Global Neonatal & Children's Health 2
Session: Global Neonatal & Children's Health 2
745 - Predictors of poor outcome in children presenting with non-malarial fever in rural Malawi
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 745.6157
Christina E. Stiles, UCSF Benioff Children's Hospital Oakland, Emeryville, CA, United States; Gama Petulo. Bandawe, Malawi University of Science and Technology, Blantyre, Blantyre, Malawi; Lameck Lawson. Khonde, University of California, San Francisco, School of Medicine, Blantyre city, Blantyre, Malawi; Pious Clifford. Makaka, St Joseph's College of Health Sciences, The Catholic University of Malawi, Blantyre, Blantyre, Malawi; Teresa Kortz, University of California, San Francisco, School of Medicine, San Francisco, CA, United States; Newton Addo, University of California, San Francisco, School of Medicine, San Francisco, CA, United States; Kimberly Baltzell, University of California San Francisco, San Francisco, CA, United States; Phil Rosenthal, UCSF, SAN FRANCISCO, CA, United States; Anneka Hooft, University of California, San Francisco, School of Medicine, San Franciscio, CA, United States

Christina E. Stiles, MD (she/her/hers)
Resident Physician
UCSF Benioff Children's Hospital Oakland
Emeryville, California, United States
Presenting Author(s)
Background: Despite the high burden of pediatric morbidity and mortality in sub-Saharan Africa, few studies have assessed predictors of poor outcomes in children with acute non-malarial fever. Diagnostic testing in these settings is limited, and clinicians must rely on clinical signs using the World Health Organization’s Integrated Management of Childhood Illness (IMCI) guidelines for decision-making. As point-of-care (POC) biomarker tests, namely C-reactive protein (CRP) and procalcitonin (PCT), become more widely available, further evaluation of their utility as clinical adjuncts for risk prediction is warranted.
Objective: To characterize the predictive ability of IMCI danger signs and POC biomarkers for poor outcomes in children presenting with acute, non-malarial fever in a public hospital in rural Malawi.
Design/Methods: We enrolled children (>28 days to ≤5 years of age) presenting with non-malarial fever (measured or reported) to the under-five pediatric outpatient and inpatient departments at Thyolo District Hospital (March 7-September 18, 2024). We collected clinical, socio-behavioral, and laboratory data on enrollment. Disposition and outcomes were collected on day of discharge (if admitted) and Day 28. Primary outcome was a composite of poor outcomes including death, readmission, or revisit by Day 28.
Results: There were a total of 126 outpatients and 213 admitted children with follow-up completed at Day 28. Of those admitted, pneumonia was the most common diagnosis (n=152, 45%) followed by other respiratory infection (n=70, 21%) and sepsis (n=61,18%). Antibiotics were given to 89% of participants (n=303). There were 50 participants with the primary outcome: 3 deaths, 10 readmissions, and 45 revisits. For all participants, CRP was the only factor significantly associated with the primary outcome (p=0.002, Table 1). For this outcome, Area Under the Receiver Operating Characteristics Curve (AUROC) was 0.63 for CRP and 0.56 for PCT (Figure 1). A CRP of 23.5mg/L (ROC peak) had a Negative Predictive Value (NPV) of 0.87 (95% CI=0.82-0.91), similar to the NPV of the presence of any danger sign (0.85, 95% CI=0.80-0.89). Combining this CRP cutoff with any danger sign increased sensitivity with minimal effect on NPV (Table 2).
Conclusion(s): Frequency of antibiotic use in children evaluated for non-malarial febrile illness was high. Danger signs, CRP, and PCT had comparable NPV for poor outcome. Sensitivity was low overall but increased when combining biomarkers with any danger sign. More studies are needed to determine the role of biomarkers for clinical risk prediction in pediatric non-malarial fever.
Table 1
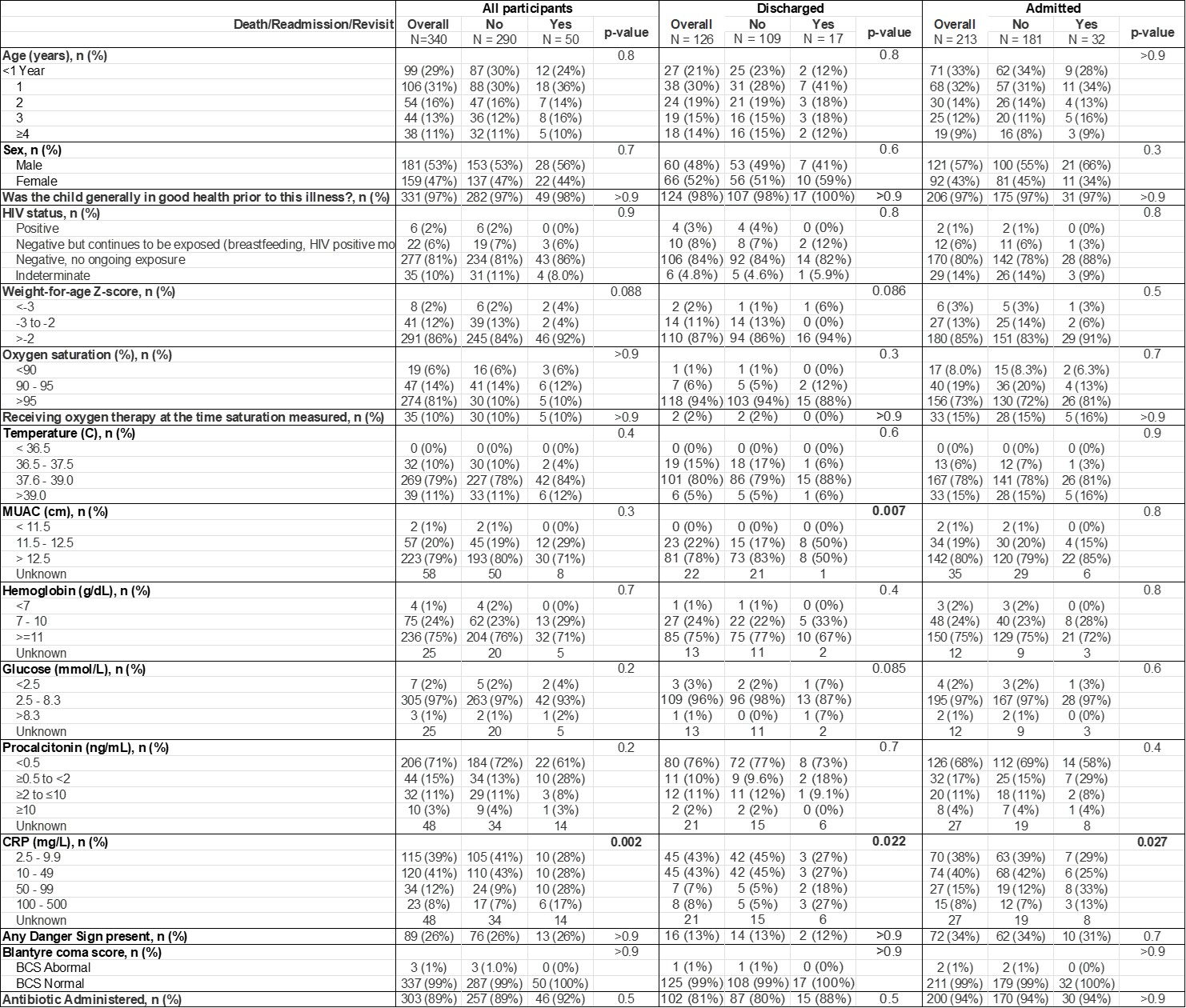 Demographic and clinical data of participants, stratified by initial disposition. Fisher’s exact test and Pearson’s Chi-squared test were utilized to determine statistical significance with a cutoff of p<0.05
Demographic and clinical data of participants, stratified by initial disposition. Fisher’s exact test and Pearson’s Chi-squared test were utilized to determine statistical significance with a cutoff of p<0.05Figure 1
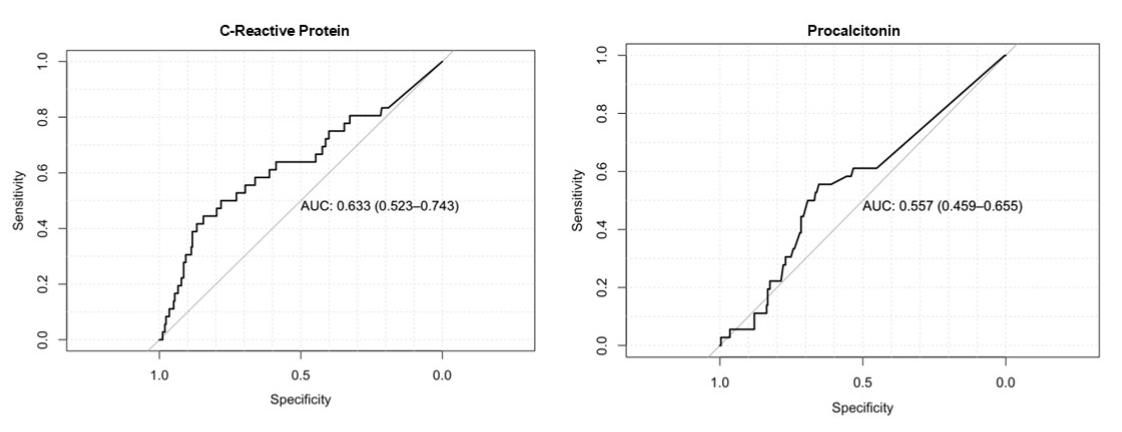 Receiver operating characteristic curves for CRP and PCT biomarkers for the primary outcome of death, readmission, or revisit
Receiver operating characteristic curves for CRP and PCT biomarkers for the primary outcome of death, readmission, or revisitTable 2
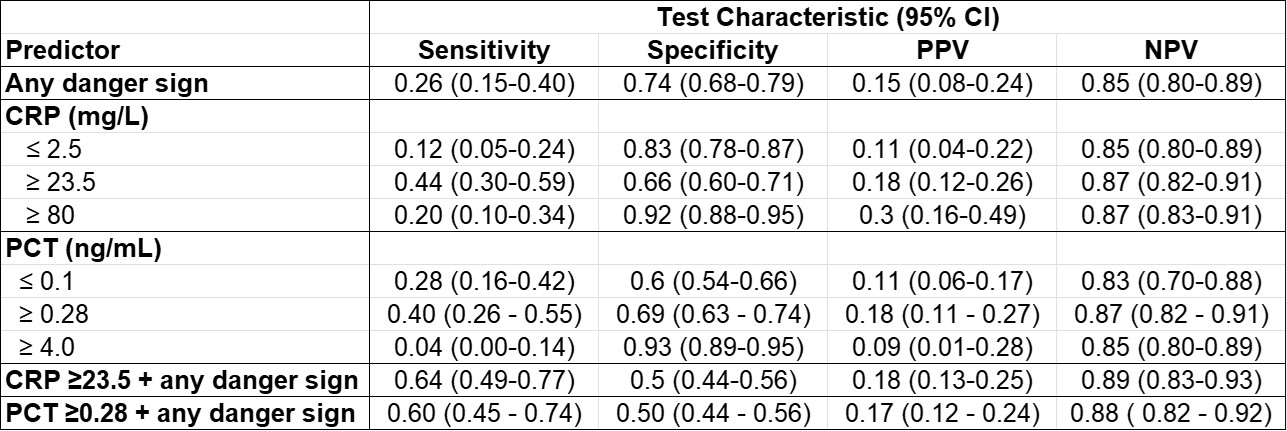 Test characteristics for any danger sign, CRP and PCT for the primary outcome of death, readmission, or revisit
Test characteristics for any danger sign, CRP and PCT for the primary outcome of death, readmission, or revisitTable 1
 Demographic and clinical data of participants, stratified by initial disposition. Fisher’s exact test and Pearson’s Chi-squared test were utilized to determine statistical significance with a cutoff of p<0.05
Demographic and clinical data of participants, stratified by initial disposition. Fisher’s exact test and Pearson’s Chi-squared test were utilized to determine statistical significance with a cutoff of p<0.05Figure 1
 Receiver operating characteristic curves for CRP and PCT biomarkers for the primary outcome of death, readmission, or revisit
Receiver operating characteristic curves for CRP and PCT biomarkers for the primary outcome of death, readmission, or revisitTable 2
 Test characteristics for any danger sign, CRP and PCT for the primary outcome of death, readmission, or revisit
Test characteristics for any danger sign, CRP and PCT for the primary outcome of death, readmission, or revisit
