Global Neonatal & Children's Health 2
Session: Global Neonatal & Children's Health 2
747 - Randomized Controlled Trial of Intrapartum Azithromycin in Asia and Africa: Regional Differences in the Neurodevelopmental Follow-up Study of the A-PLUS trial
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 747.6369
Manimaran Ramani, University of Alabama School of Medicine, Mobile, AL, United States; Elizabeth McClure, RTI International, Chapel Hill, NC, United States; Jennifer J.. Hemingway-Foday, RTI International, Research Triangle Park, NC, United States; Kathryn Gustafson, Duke University School of Medicine, Durham, NC, United States; Roger B.. Williams, RTI International, Durham, NC, United States; Marissa Trotta, RTI International, Hillsborough, NC, United States; Cecilia Rasco, RTI International, Durham, NC, United States; Waldemar Carlo, UAB School of Medicine, Birmingham, AL, United States

Manimaran Ramani, MD
Chief Medical Officer
University of Alabama School of Medicine
Mobile, Alabama, United States
Presenting Author(s)
Background: Long-term neurodevelopmental impairment in survivors of birth asphyxia is a major burden worldwide. Differences in medical practices, cultural practices, resources, and the prevalence of sepsis can variably impact the neurodevelopmental outcomes of asphyxiated infants in different regions of the world. In a large randomized control trial, Azithromycin Prevention in Labor Use Study (A-PLUS), intrapartum azithromycin reduced maternal mortality and morbidity but did not reduce neonatal mortality. However, since azithromycin has also been shown to reduce brain injury in preclinical models of asphyxia, it is relevant to evaluate neuro-developmental outcomes in infants born to pregnant women enrolled in A-PLUS.
Objective: To evaluate and assess whether there are regional differences in the effects of intrapartum azithromycin on neurodevelopmental outcomes of birth-asphyxiated infants born in Asia versus Africa.
Hypothesis: Among survivors of birth asphyxia born in Asia and Africa regions, a single 2 gram dose of intrapartum azithromycin given to laboring mothers will differentially increase the Bayley Scales of Infant Development, Third Edition (BSID-III) Cognitive Composite Score (CCS) at 24 (±1) months corrected age compared to children born to mothers exposed to placebo.
Design/Methods: This is a neurodevelopmental follow-up study of ≥ 34 weeks asphyxiated (Apgar score of < 7 at 5 minutes and/or requiring positive pressure ventilation at birth) born in the A-PLUS trial. The study was conducted in 5 sites (India [2 sites], Pakistan, Zambia, and Democratic Republic of Congo). BSID-III and Ages &Stages Questionnaire-3 (ASQ-3) were done at 24 (±1) months corrected age. A generalized linear model was used to control for site and baseline characteristics.
Results: 350 were enrolled and completed the study (Figure 1). Baseline characteristics were similar between the groups and regions (Table 1). CCS did not differ between by treatment or region (mean [SD], Africa: Azithromycin; 90.2 [13.7], vs. Placebo 90.7 [13.5], mean difference, [95% CI; 0.57 [-3.43, 4.56], Asia: Azithromycin; 90.4 [10.4], vs. Placebo 90.9 [11.3], mean difference, [95% CI; -0.79 [-4.33, 2.74]). The components of BSID-III and the total scores of ASQ-3 did not differ between groups (Table 2).
Conclusion(s): A single oral dose of azithromycin administered to laboring mothers who gave birth to asphyxiated infants did not differentially affect neurodevelopmental outcomes between infants born in Asia and Africa.
Figure 1
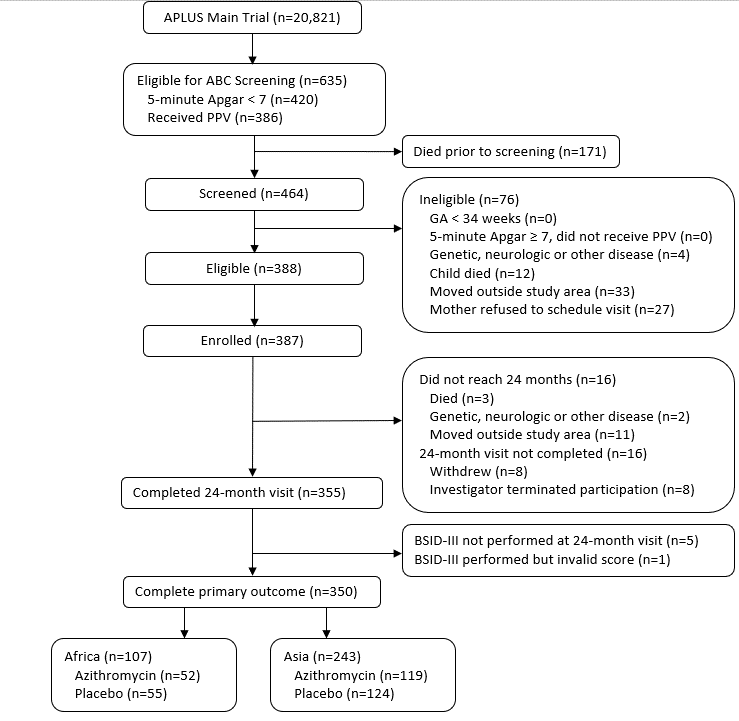 CONSORT Diagram
CONSORT DiagramTable 1. Participant Characteristics at APLUS Enrollment and 2-Year Follow-up, by Treatment and Region
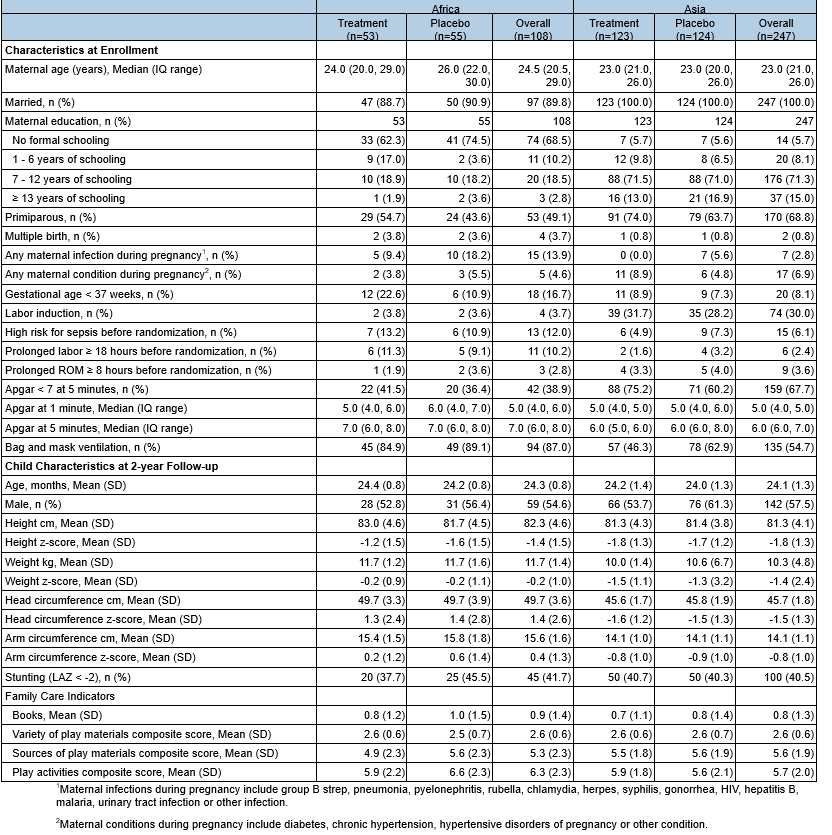 1 Maternal infections during pregnancy include group B strep, pneumonia, pyelonephritis, rubella, chlamydia, herpes, syphilis, gonorrhea, HIV, hepatitis B, malaria, urinary tract infection or other infection.
1 Maternal infections during pregnancy include group B strep, pneumonia, pyelonephritis, rubella, chlamydia, herpes, syphilis, gonorrhea, HIV, hepatitis B, malaria, urinary tract infection or other infection.2 Maternal conditions during pregnancy include diabetes, chronic hypertension, hypertensive disorders of pregnancy or other condition.
Table 2. Outcomes by Treatment and Region
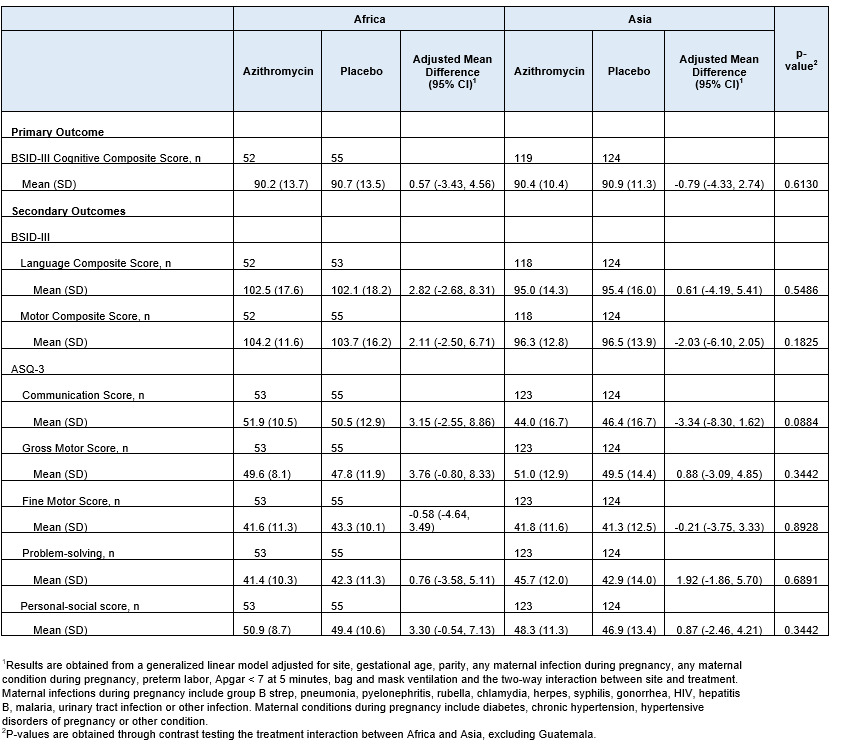 1 Results are obtained from a generalized linear model adjusted for site, gestational age, parity, any maternal infection during pregnancy, any maternal condition during pregnancy, preterm labor, Apgar < 7 at 5 minutes, bag and mask ventilation and the two-way interaction between site and treatment. Maternal infections during pregnancy include group B strep, pneumonia, pyelonephritis, rubella, chlamydia, herpes, syphilis, gonorrhea, HIV, hepatitis B, malaria, urinary tract infection or other infection. Maternal conditions during pregnancy include diabetes, chronic hypertension, hypertensive disorders of pregnancy or other condition.
1 Results are obtained from a generalized linear model adjusted for site, gestational age, parity, any maternal infection during pregnancy, any maternal condition during pregnancy, preterm labor, Apgar < 7 at 5 minutes, bag and mask ventilation and the two-way interaction between site and treatment. Maternal infections during pregnancy include group B strep, pneumonia, pyelonephritis, rubella, chlamydia, herpes, syphilis, gonorrhea, HIV, hepatitis B, malaria, urinary tract infection or other infection. Maternal conditions during pregnancy include diabetes, chronic hypertension, hypertensive disorders of pregnancy or other condition.2 P-values are obtained through contrast testing the treatment interaction between Africa and Asia, excluding Guatemala.

