Immunizations/Delivery 2
Session: Immunizations/Delivery 2
690 - RSV Protection for Neonates Before Hospital Discharge
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 690.6310
Jessica Wilcox, Pennsylvania Hospital, Exton, PA, United States; Kelly Wade, Children's Hospital of Philadelphia, Philadelphia, PA, United States; Elizabeth Faville, Perelman School of Medicine at the University of Pennsylvania, Middletown, DE, United States; Sagori Mukhopadhyay, Childrens Hospital of Philadelphia, Philadelphia, PA, United States
- KW
Kelly Wade, MD PhD MSCE (she/her/hers)
Professor of Clinical Pediatrics
Perelman School of Medicine at the University of Pennsylvania
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Respiratory syncytial virus (RSV) is the leading cause of infant hospitalization. Just prior to 2023-2024 RSV season, the ACIP recommended passive immunization for newborns by either postnatal injection of nirsevimab-alip or RSV vaccination in pregnancy. Most birthing hospitals did not implement nirsevimab-alip for all newborns
Objective: To describe an implementation process enabling routine nirsevimab-alip administration at an urban tertiary perinatal center. To determine the proportion of discharged neonates with RSV protection between Nov 2023 - Mar 2024.
Design/Methods: We describe our nirsevimab-alip implementation process using the Consolidated Framework for Implementation Research domains (Table 1). The primary outcome was the proportion of neonates discharged with RSV protection, defined as either pregnancy RSV vaccine ≥14 days before birth or postnatal nirsevimab-alip, and stratified by neonatal setting (ICU vs WELL) and insurance status (Vaccine for Children (VFC) vs commercial insurance (INSUR). Group comparison by Chi-squared test. Receipt of hepatitis B vaccine was a balance measure.
Results: Implementation was supported by institutional culture and required drug addition to inpatient formulary, VFC enrollment, pharmacy IT and infrastructure to allocate VFC or INSUR nirsevimab-alip stock to appropriate infants, and multidisciplinary education (Table 1). Implementation occurred in two phases (Table 2). The first phase (Nov 2023 - Jan 2024) was focused in the ICU, with 147 discharges (24-41 weeks gestation, birth weight 585-4579g) among whom 78% had RSV protection, primarily nirsevimab-alip. The second phase (Feb-March 2024) included all 780 discharged neonates, among whom 77% had RSV protection (nirsevimab-alip 45% and pregnancy RSV vaccine 32%). RSV protection increased over time (Figure 1A). In Feb-March, RSV protection was significantly more common in neonates discharged from ICU (83/93, 83%) compared to WELL (521/687, 76%) and neonates with INSUR (395/490, 81%) compared to VFC (209/290, 72%). Nirsevimab-alip was significantly more common in neonates with VFC while pregnancy RSV vaccine more common in neonates with INSUR (Figure 2B, Table 2). VFC provided 51% of the nirsevimab-alip doses. Hepatitis B vaccination rate remained >80% over time.
Conclusion(s): Within months of ACIP recommendations, our birthing hospital enrolled in VFC and achieved RSV protection in 77% of discharged neonates. RSV protection among infants with VFC was primarily dependent on nirsevimab-alip. Birth hospitals offering nirsevimab to all infants can protect the most vulnerable while promoting equitable RSV protection.
Table 1
.jpg)
Table 2
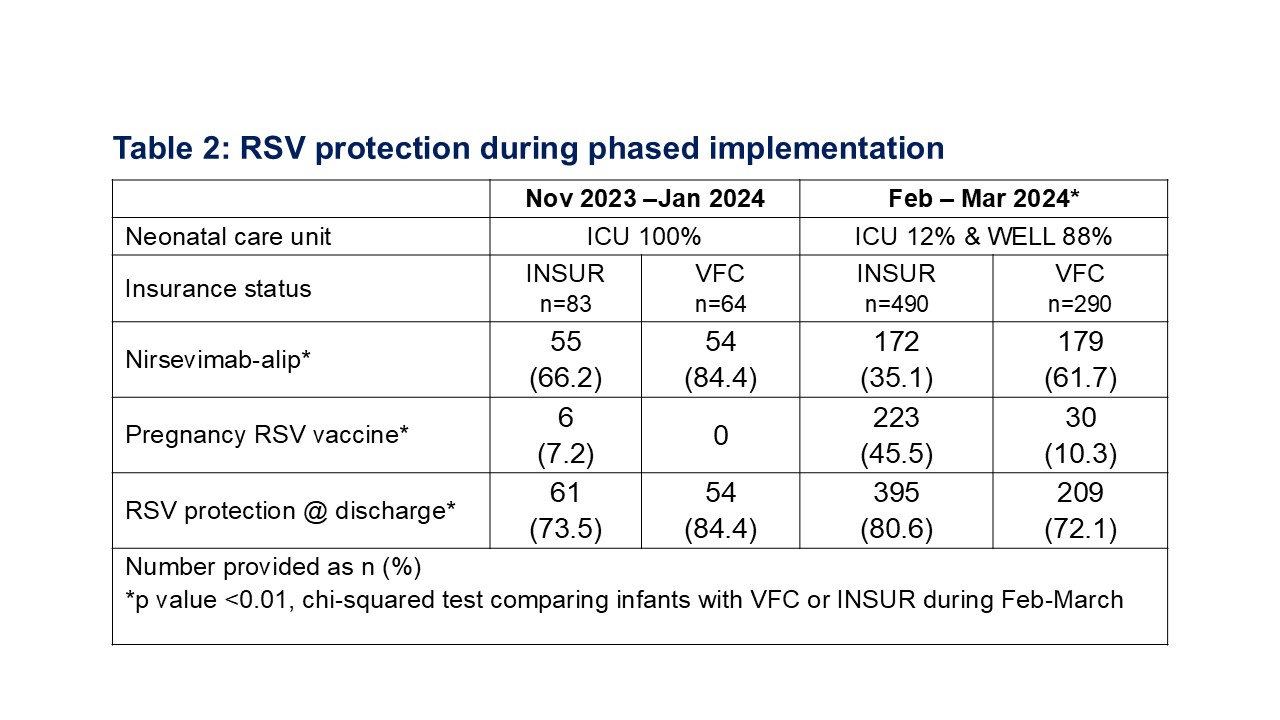
Figure 1