Neonatal Pulmonology - Basic/Translational Science 1
Session: Neonatal Pulmonology - Basic/Translational Science 1
216 - Right ventricular structure and intrapulmonary arteriolar muscularization are unaffected by physiological treatment with rhIGF-1 complex in mechanically ventilated preterm lambs
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 216.6282
Matthew S. Douglass, University of Utah School of Medicine, Salt Lake City, UT, United States; Bryce Heslop, University of Utah School of Medicine, MIDVALE, UT, United States; Andrew Rebentisch, University of Utah School of Medicine, Salt Lake CIty, UT, United States; Jakob Van Boerum, University of Utah Health, Salt Lake City, UT, United States; Yongmei Zhang, University of Utah, West Valley City, UT, United States; Keana A. Estorpe, University of Utah School of Medicine, Salt Lake City, UT, United States; Mar Janna Dahl, University of Utah School of Medicine, Salt Lake City, UT, United States; Donald M. Null, University of Utah School of Medicine, Sacramento, CA, United States; Victoria Niklas, Oak Hill Bio Ltd, Beverly Hills, CA, United States; Mark T. McHale, Oak Hill Bio Ltd, Burton on the Wolds, England, United Kingdom; Robert M. Ward, University of Utah School of Medicine, Salt Lake City, UT, United States; Norman W. Barton, Oak Hill Bio, Phoenix, MD, United States; Kurt H.. Albertine, University of Utah, Salt Lake City, UT, United States

Matthew S. Douglass, DO
Assistant Professor
University of Utah School of Medicine
Salt Lake City, Utah, United States
Presenting Author(s)
Background: Insulin-like growth factor 1 (IGF-1) is an important growth factor involved in heart and lung development. IGF-1 plasma protein levels decrease dramatically after preterm birth. Restoring IGF-1 to physiological levels after preterm birth may improve pulmonary outcomes. We showed that physiological replacement of IGF-1 increases alveolar capillary growth and pulmonary function in mechanically ventilated preterm lambs (Hubbard, E-PAS2023:239.339). However, the impact of physiologic replacement of IGF-1 on the postnatal preterm heart is unknown. In other neonatal in vitro and in vivo animal models, IGF-1 signaling promoted arterial muscularization, cardiomyocyte (CM) hypertrophy, and myocardial fibrotic pathways.
Objective: Our objective was to determine whether continuous intravenous (IV) infusion of recombinant human (rh) IGF-1 complexed with binding protein 3 (rhIGF-1 complex) led to CM hypertrophy, fibrosis, and angiogenesis in the right ventricle (RV) or increased muscularization of intrapulmonary arterioles (PA) in the mechanically ventilated preterm lambs.
Design/Methods: Preterm lambs at 128d gestation were exposed to antenatal steroids, treated with surfactant and caffeine, and supported by invasive mechanical ventilation for 7d. The control group received vehicle (IGF-1 diluent in saline; n=10; 5F 5M). The treated group received rhIGF-1 complex (optimized dose of 1.5 mg/kg/d IV; n=10; 5F 5M). Cardiac tissue was available for subsets of both groups (vehicle n=7; 5F 2M and rhIG1-complex n=7; 4F 3M). RV sections from the mid-ventricular level were fixed. Peripheral lung tissue sections were fixed to analyze PA muscularization at the level of terminal bronchioles. Immunohistochemistry and immunofluorescence methods were used to measure CM cross-sectional area, capillary surface density, and fibrosis in the RV, and PA diameter, PA smooth muscle (SMC) layer thickness and area.
Results: No statistically significant differences were detected for RV CM cross-sectional area, capillary surface density, or fibrosis. Likewise, no statistically significant differences were detected for PA diameter or SMC layer thickness or area. Sex differences were not assessed because sex distribution was unequal for the available cardiac tissue.
Conclusion(s): We conclude that RV structure and PA muscularization are unaffected by physiological replacement of IGF-1 protein in plasma, by continuous IV infusion of rhIGF-1 complex, in mechanically ventilated preterm lambs. These findings support that physiological replacement of IGF-1 after preterm birth improves lung development without negatively impacting heart development.
Table 1
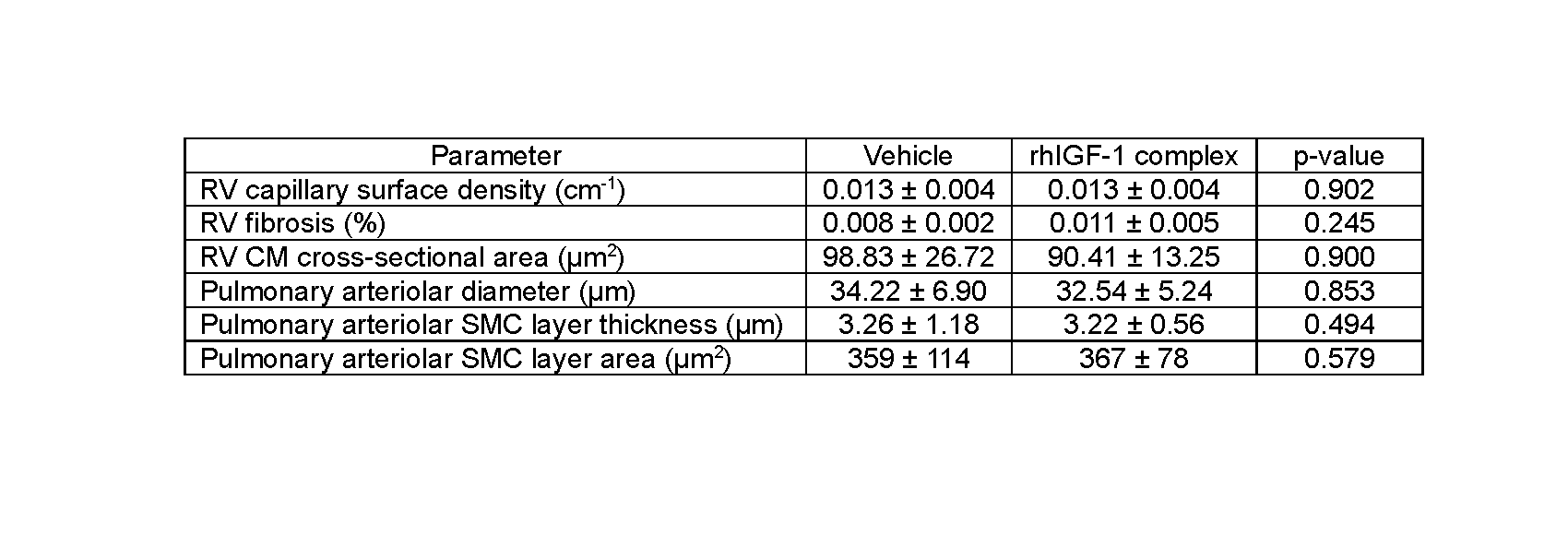 Right ventricular (RV) and pulmonary arteriolar structural measurements (mean ± SD) in mechanically ventilated preterm lambs receiving continuous infusion of vehicle or rhIGF-1 complex. Statistical analysis did not detect significant differences between the two groups of preterm lambs (Mann-Whitney U test).
Right ventricular (RV) and pulmonary arteriolar structural measurements (mean ± SD) in mechanically ventilated preterm lambs receiving continuous infusion of vehicle or rhIGF-1 complex. Statistical analysis did not detect significant differences between the two groups of preterm lambs (Mann-Whitney U test).
