Genomics/Epigenomics 2
Session: Genomics/Epigenomics 2
404 - Sex-specific Molecular Pathways in Neonates Exposed to Maternal Opioid Use
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 404.6727
Tomoko Kaneko-Tarui, Tufts University School of Medicine, Boston, MA, United States; Kiran Singh, Tufts Medical Center, Boston, MA, United States; Francesca Carasi-Schwartz, Tufts Medical Center, Boston, MA, United States; Kelsea Gildawie, Simmons University, Boston, MA, United States; Fair M.. Vassoler, Tufts University, Grafton, MA, United States; Elizabeth M. Byrnes, Tufts University, North Grafton, MA, United States; Elizabeth Yen, Tufts University School of Medicine, Boston, MA, United States

Tomoko Kaneko-Tarui, MD., PhD. (she/her/hers)
Assistant Professor
Tufts University School of Medicine
Boston, Massachusetts, United States
Presenting Author(s)
Background: Maternal opioid use affects male and female offspring differently, though mechanisms remain unclear. Our preliminary data suggest altered reward signaling may drive these sex differences, with increased dopamine receptor type 2 (DRD2) expression in opioid-exposed males. Animal studies indicate inflammation enhances opioid reward signaling via opioid binding to the microglia's toll-like receptor 4 (TLR4), initiating a pro-inflammatory cascade.
Objective: To investigate sex-specific molecular effects of maternal opioid exposure on inflammatory and hypothalamic/reward pathways.
Design/Methods: Saliva samples from nine opioid-exposed and nine non-exposed neonates were collected within 48 hours of birth and analyzed, focusing on reward, energy homeostasis, inflammation, oxidative stress, and neuropathology pathways. Continuous data were analyzed using a Student’s t-test and categorical data using a Chi-square test.
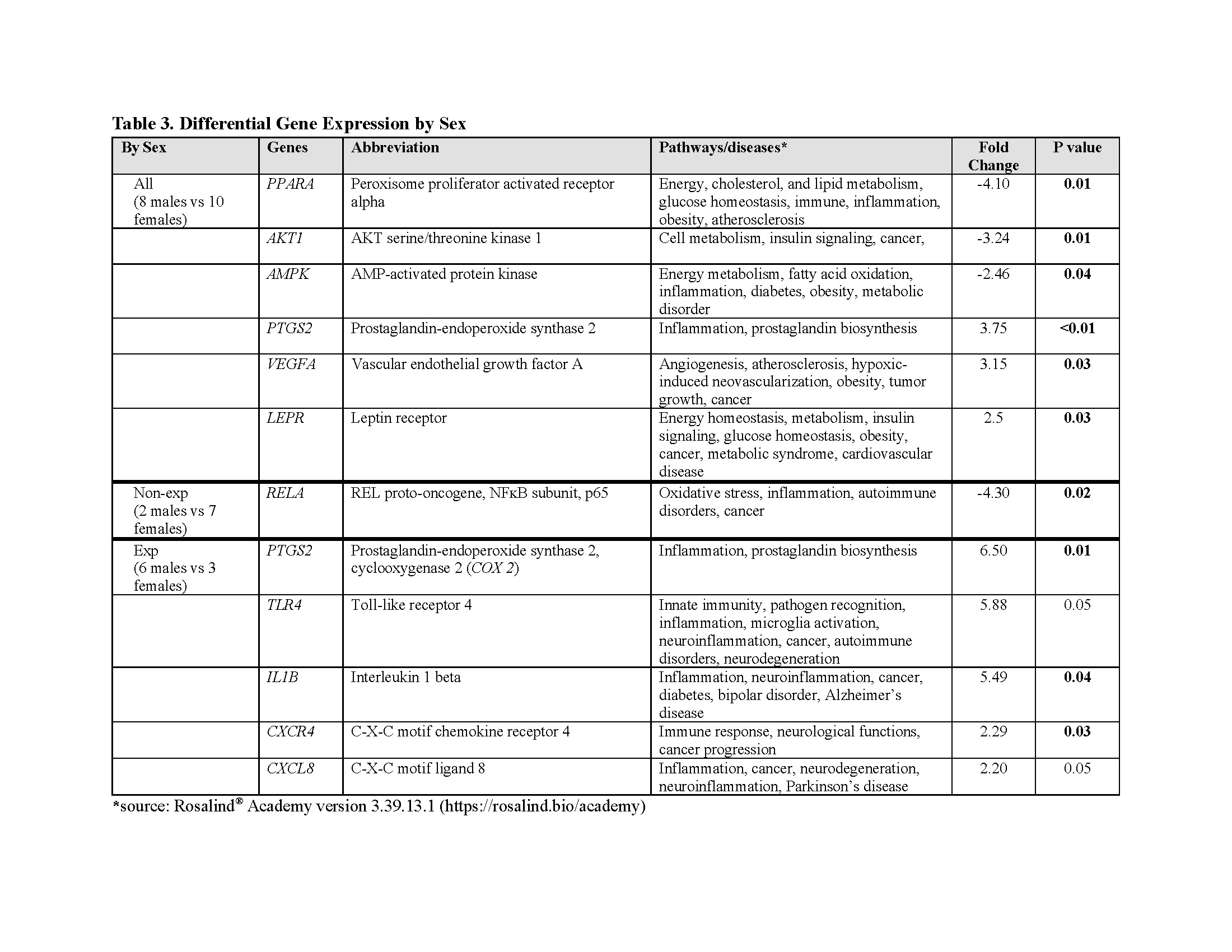
Results: Opioid-exposed neonates are born significantly smaller, with a greater incidence of small for gestational age and maternal hepatitis C than in the non-exposed cohort (Table 1). Gene analyses revealed distinct molecular pathways linked to maternal opioid use and sex differences. Stratified by exposure, opioid-exposed females showed lower inflammation-related gene expression than non-exposed females. Conversely, opioid-exposed males exhibit elevated oxidative stress, inflammation, and DRD2 gene expression compared to non-exposed males. Methadone exposure was associated with greater immune- and neuro-related genes than buprenorphine (Table 2). Stratified by sex, non-exposed males had lower oxidative stress and inflammation than non-exposed females, while opioid-exposed males showed upregulation in energy/metabolism, inflammation, and TLR4 genes relative to opioid-exposed females (Table 3).
Conclusion(s): Maternal opioid use is associated with unique sex-specific molecular pathways, with heightened inflammation in opioid-exposed males and reduced inflammation in opioid-exposed females. The upregulation of DRD2 and TLR4 genes in opioid-exposed males highlights inflammation’s role in opioid reward signaling through TLR4 modulation. Given the pronounced energy homeostasis and metabolic themes in the exposed cohort and their reduced birth size, future studies should assess the impact of maternal opioid use on offspring growth and metabolic health.
Table 1. Demographic Data
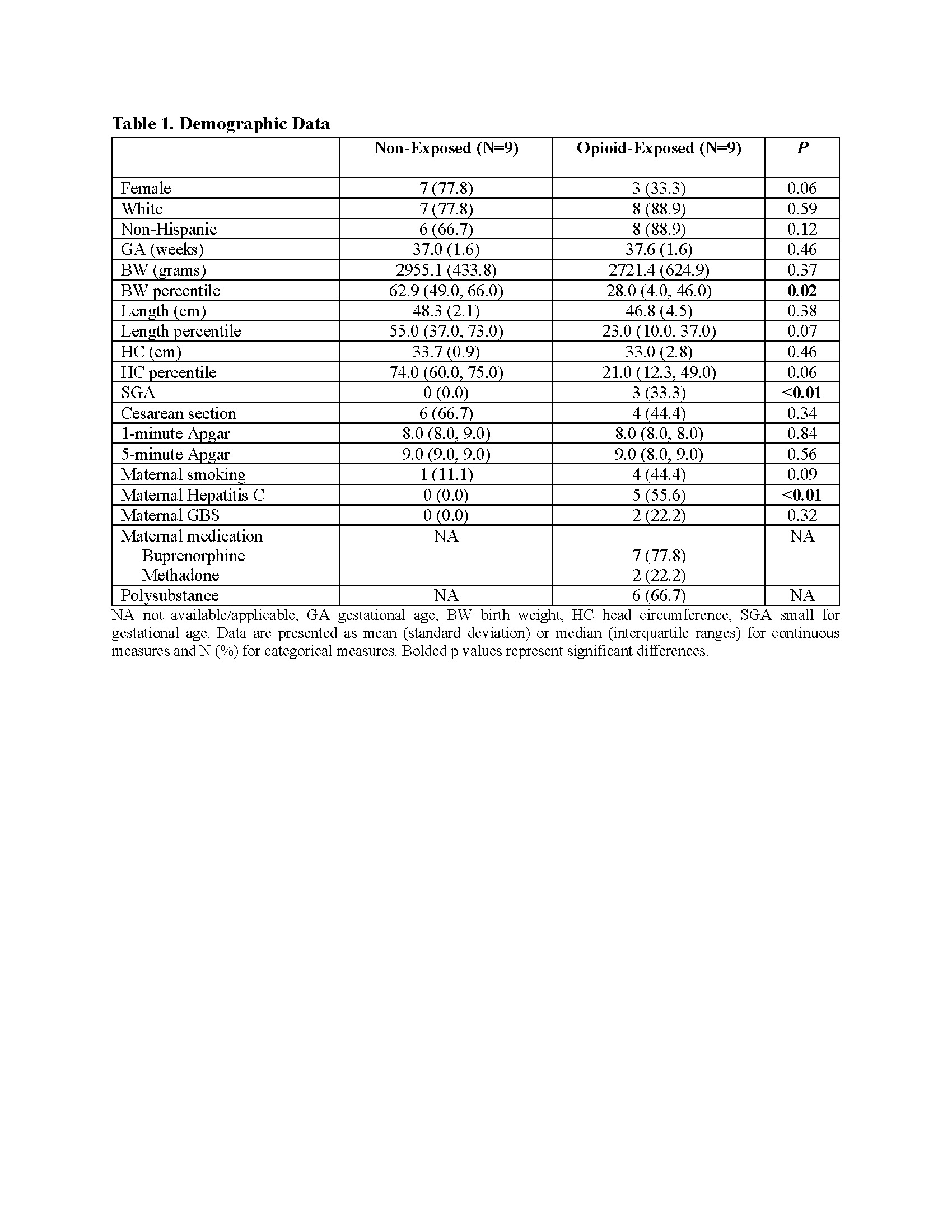
Table 2. Differential Gene Expression by Exposure
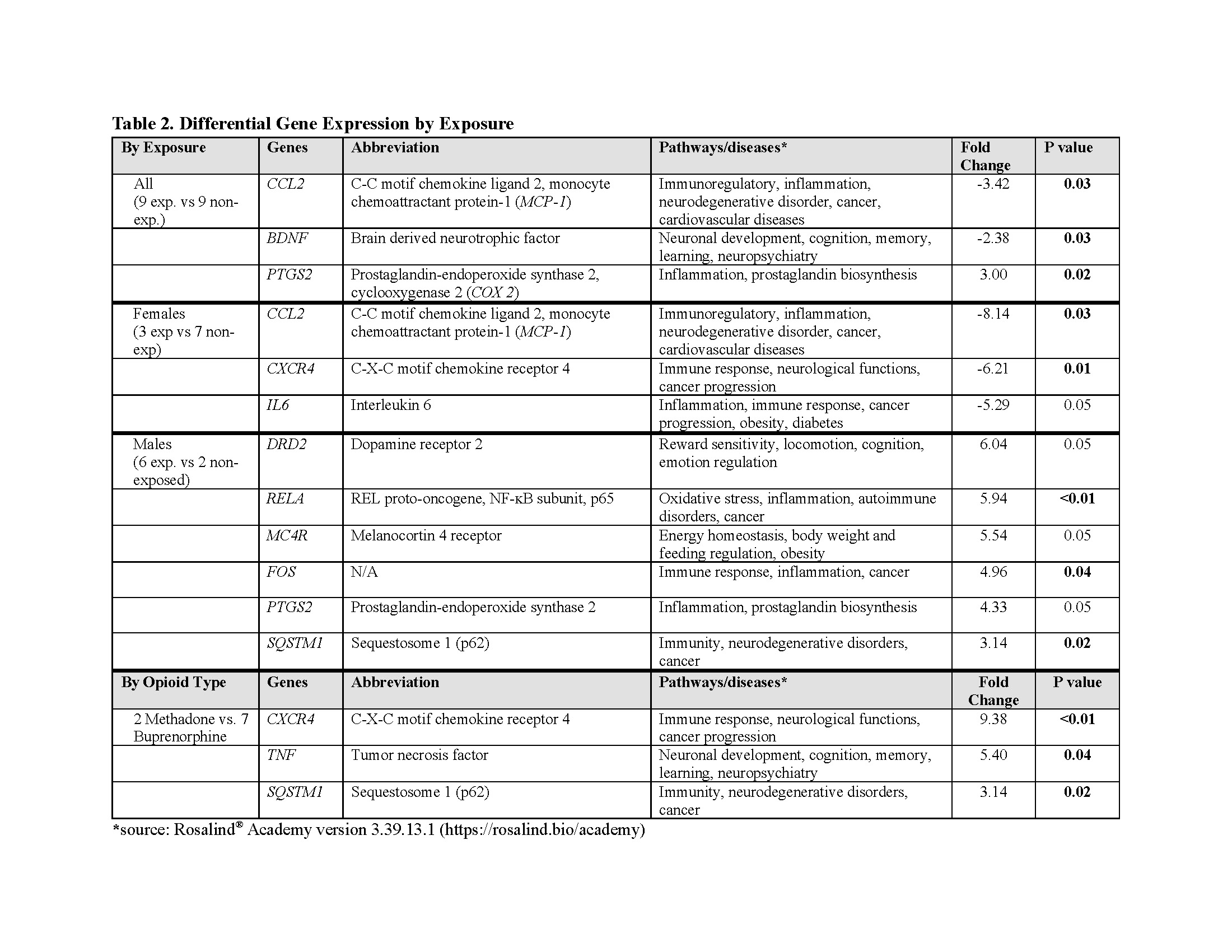
Table 3. Differential Gene Expression by Sex