Immunizations/Delivery 2
Session: Immunizations/Delivery 2
679 - Changing the Age of Routine HPV Vaccination Initiation to 9-10 years: Experiences of Clinicians
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 679.4112
Allison Kempe, University of Colorado AMC, ACCORDS, aurora, CO, United States; Sean T. O'Leary, University of Colorado School of Medicine, Denver, CO, United States; Brenda L. Beaty, University of Colorado, Aurora, CO, United States; Sitaram Vangala, University of California, Los Angeles David Geffen School of Medicine, Los Angeles, CA, United States; Alison Saville, University of Colorado School of Medicine, Aurora, CO, United States; Dennis Gurfinkel, University of Colorado Anschutz Medical Campus, Aurora, CO, United States; Christina S.. Albertin, University of Rochester School of Medicine and Dentistry, Indianapolis, IN, United States; Emma J. Clark, University of California, Los Angeles David Geffen School of Medicine, Venice, CA, United States; Peter Szilagyi, UCLA, Los Angeles, California, CA, United States

Allison Kempe, MD, MPH (she/her/hers)
professor of pediatrics
University of Colorado AMC
aurora, Colorado, United States
Presenting Author(s)
Background: Highly effective and safe HPV vaccines have been available since 2006, but are vastly underutilized, with HPV series completion rates of only 46% by 13 yr. olds nationally. HPV vaccine is routinely recommended by ACIP at ages 11-12 but may be given as early as 9-10 yrs. Data suggest the vaccine has high immunogenicity at 9-10 yrs. with little waning. However, scant data exist about experiences of clinicians who have changed the age of routine initiation from 11-12 yrs. to 9-10 yrs.
Objective: To compare the experiences of clinicians who are routinely recommending HPV vaccine at age 11-12 yrs. to those who have switched to recommending at age 9-10 yrs. with respect to 1) parental concerns, 2) parental compliance with recommendations and 3) length of discussion times.
Design/Methods: We conducted a clinician survey as part of an ongoing cluster randomized trial in which we randomized pediatric practices to Intervention (switch to initiation at 9-10 yrs.) versus Control (continue current HPV vaccine initiation at ages 11-12 yrs.). We included 17 practices (9 Intervention) in CO and 16 practices (8 Intervention) in CA. We surveyed all clinicians in both Intervention and Control practices 30 mos. after initiation of the trial.
Results: The survey response rate was 94% (101/108) in Intervention and 86% (121/141) in Control practices. Responses did not differ significantly between Intervention and Control groups with respect to how often clinicians were experiencing concerns or pushback regarding HPV vaccine, long discussion times or poor compliance with vaccine recommendations (Figure 1). Significantly more clinicians in the Intervention group reported that the amount of time they were spending discussing the first dose of HPV had decreased since switching to earlier introduction than in the Control group over the same time period.
Conclusion(s): Clinicians who switched to recommending HPV vaccination at ages 9-10 (Intervention group) reported similar rates of parental concerns and compliance with their HPV vaccination recommendations compared with clinicians who continued to recommend initiation at ages 11-12, but those who have switched to initiation at age 9-10 perceived a decrease in the amount of time they were spending on discussions of the first HPV dose compared to those who have continued to recommend at 11-12 yrs. Initiating at 9-10 yrs. was perceived by clinicians to be just as acceptable to parents as initiating at 11-12 yrs. Results of the completed trial will determine if initiating HPV vaccination earlier increases vaccination completion rates by age 13 yrs overall.
Comparisons of Clinician Experiences with Parental Acceptance of HPV Vaccination Between Study Arms
.jpg) Figure 1
Figure 1Comparisons of Time Clinicians Spend Discussing the First HPV Vaccine Dose Between Study Arms
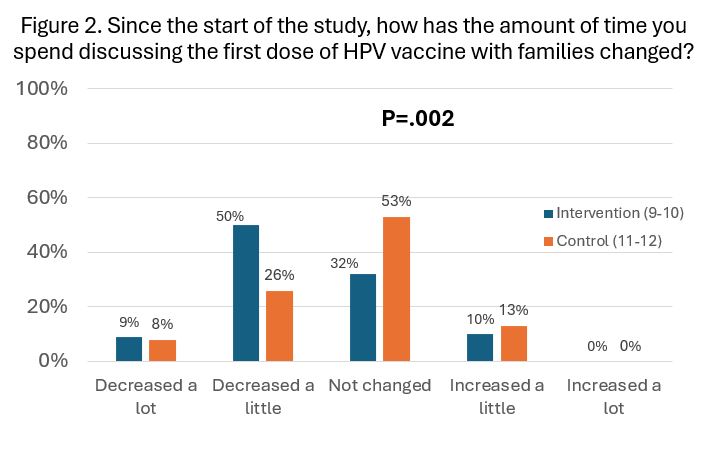 Figure 2
Figure 2Comparisons of Clinician Experiences with Parental Acceptance of HPV Vaccination Between Study Arms
.jpg) Figure 1
Figure 1Comparisons of Time Clinicians Spend Discussing the First HPV Vaccine Dose Between Study Arms
 Figure 2
Figure 2
