Neonatal Hemodynamics and Cardiovascular Medicine 1
Session: Neonatal Hemodynamics and Cardiovascular Medicine 1
194 - Exploring the Efficacy of Indomethacin and Ibuprofen in Male and Female Premature Infants
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 194.4911
Priya R. Gupta, University of Connecticut School of Medicine, Farmington, CT, United States; Mariann Pappagallo, CCMC at U CONN Health, Farmington, CT, USA, CT, United States; Ted Rosenkrantz, Connecticut Children's Medical Center, Farmington, CT, United States; Leonard Eisenfeld, University of Con, West Hartford, CT, United States; Naveed Hussain, Connecticut Children's Medical Center, Farmington, CT, United States
- PG
Priya R. Gupta
Medical Student
University of Connecticut School of Medicine
Farmington, Connecticut, United States
Presenting Author(s)
Background: In very-low-birthweight (VLBW) infants (birth weight < 1500 g), the prevalence of patent ductus arteriosus (PDA) varies from 18% to 77%. For the past two decades, the most commonly used medications for medical management of PDA were indomethacin and ibuprofen. Both indomethacin and ibuprofen can have side effects, such as renal failure and gastrointestinal bleeding, and treatment failure occurs in 20-40% of infants. One factor that may influence success rates is infant sex. Previous small studies have found that male infants were more likely to have a successful ductal closure in response to indomethacin. However, to our knowledge, there are currently no studies that have evaluated the sex-based efficacy of indomethacin and ibuprofen for PDA treatment.
Objective: To determine if infant sex impacts the efficacy of indomethacin and ibuprofen in treating PDA in premature infants.
Design/Methods: Retrospective data from VLBW infant admissions from 2011-2023 in one NICU was evaluated. The study groups included infants with gestational age (GA) 32 weeks or less who were treated for PDA with either indomethacin or ibuprofen. Failure of initial medical treatment was defined as the need for PDA ligation or the need for a repeat course of medication. Chi-squared tests were used to analyze differences between groups.
Results: Of the 808 VLBW < 33 wk. GA infants admitted, 293 infants (161 males, 132 females) were diagnosed with PDA. Of these, 195 infants (101 males, 94 females) were initially treated for PDA with either indomethacin (n=92) or ibuprofen (n= 103). Demographic and other characteristics are shown in Table 1.
Overall, treatment failure was 29% (57/195) with no significant difference between males (29%) and females (30%). Analyses of responses to initial medical treatment based on sex differences are shown in Table 2. There were no sex-based differences in treatment failure when either indomethacin or ibuprofen was used for initial PDA management. Multiple regression analyses were done to adjust for differences in GA, Hispanic ethnicity, and antenatal steroid use, and the results were not different.
Conclusion(s): Failure of initial medical treatment with indomethacin or ibuprofen at our center was around 30%, which is consistent with prior reports. However, based on this pilot study, no sex differences were seen in treatment response to either indomethacin or ibuprofen. Further studies are needed to explain the difference in response to treatment based on sex seen in other studies.
Table 1: Patient Characteristics
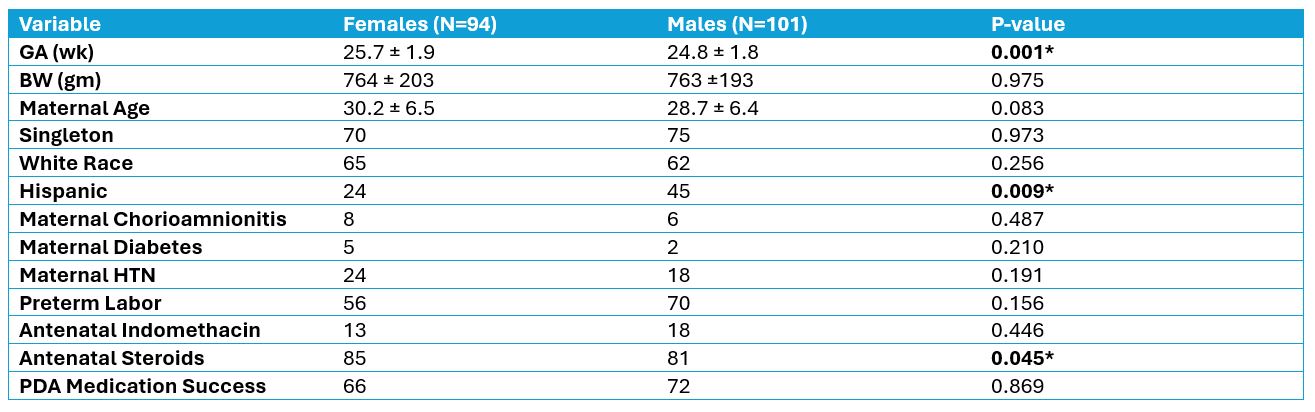 Statistical analysis using Chi-square tests and Student’s T-tests.
Statistical analysis using Chi-square tests and Student’s T-tests. Table 2: Initial PDA Medication and Treatment Outcomes Based on Patient Sex – Chi-square tests
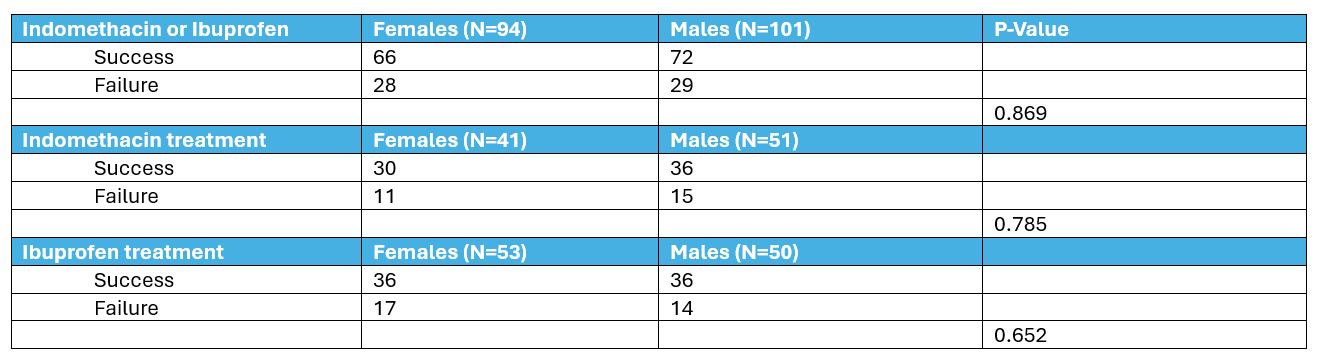 Failure of initial medical treatment was defined as the need for subsequent medical retreatment or surgical / catheter closure of PDA.
Failure of initial medical treatment was defined as the need for subsequent medical retreatment or surgical / catheter closure of PDA.Multiple regression analyses were done to adjust for differences in GA, Hispanic ethnicity, and antenatal steroid use, and the results were not different.

