Global Neonatal & Children's Health 2
Session: Global Neonatal & Children's Health 2
736 - Effect of small-quantity lipid-based nutrient supplements on nutritional status and neurodevelopmental outcomes among children born low birthweight in Zambia
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 736.4847
Nandita Perumal, University of South Carolina, Columbia, SC, United States; Daniel S.. Farrar, The Hospital for Sick Children, Toronto, ON, Canada; Doug Parkerson, Innovations for Poverty Action, Cheshire, CT, United States; Jacqueline M.. Lauer, Boston University, Boston, MA, United States; Lindsey M. Locks, Boston University, Cambridge, MA, United States; Mpela Chembe, Innovations for Poverty Action, Lusaka, Lusaka, Zambia; Savanna Henderson, Innovations for Poverty Action, Washington, DC, United States; Tamara Billima-Mulenga, Innovations for Poverty Action, Lusaka, Lusaka, Zambia; Peter C. Rockers, Boston University, Boston, MA, United States; Günther Fink, University of Basel, Basel, Basel-Stadt, Switzerland

Nandita Perumal, PhD
Assistant Professor
University of South Carolina
Columbia, South Carolina, United States
Presenting Author(s)
Background: Globally, an estimated 14.7% of all livebirths are low birthweight (LBW; < 2500 grams), with a disproportionate number residing in low- and middle-income countries. Children who are born LBW have a higher risk of mortality and morbidity, but interventions to optimize their growth and neurodevelopmental outcomes beyond the first 6 months of life are limited.
Objective: To evaluate the effect of daily small-quantity lipid nutrient supplements (SQ-LNS) on growth and neurodevelopment of children born LBW compared to normal birthweight (NBW; ≥2500 grams) at 24-36 months of age.
Design/Methods: We used secondary data from a cluster randomized controlled trial with 1:1 allocation to the SQ-LNS compared to control arm in Zambia. Participants in the trial were caregiver-child dyads (CCDs) with a child at least 6 months of age at the beginning of the intervention. CCDs were provided with a monthly supply of Nutributter+ SQ-LNS (~110 kcal nutrient-dense paste with essential fatty acids, vitamins, and minerals) for their children to consume daily. Anthropometric measures (e.g., length/height, weight), hemoglobin levels (Hemocue 801; g/dL), and neurodevelopment (Global Scaled for Early Development [GSED]) were assessed at endline when children were between 24-36 months of age. Generalized estimating equations with robust standard error accounting for clustering were used to estimate the effect of SQ-LNS on age- and sex-standardized anthropometric indices, neurodevelopmental outcomes, and anemia among children born LBW vs NBW, adjusting for caregiver and household sociodemographic factors.
Results: Of the 863 CCDs, 49% (n = 424) and 51% (n = 439) of CCDs were assigned to the SQ-LNS compared to control arm, and 12% (101/863) of children were born LBW. Children who were born LBW and received SQ-LNS had higher length/height-for-age z-scores (adjusted mean difference [aMD]: 0.57, 95%CI: 0.07, 1.08), weight-for-age z-scores (aMD: 0.52, 95%CI: 0.12, 0.91), weight-for-height z-score (aMD: 0.25, 95%CI: -0.14, 0.64), and GSED developmental z-score (aMD: 0.62, 95% CI: 0.03, 1.21), compared to NBW children who received SQ-LNS (Figure 1). Differences in magnitude, however, were not statistically significant due to small sample sizes.
Conclusion(s): This study suggests that children who are born low birthweight have a higher potential to benefit from daily SQ-LNS supplementation compared to normal birth weight children. Larger studies with adequately powered sample sizes are needed to confirm these findings.
Figure 1.
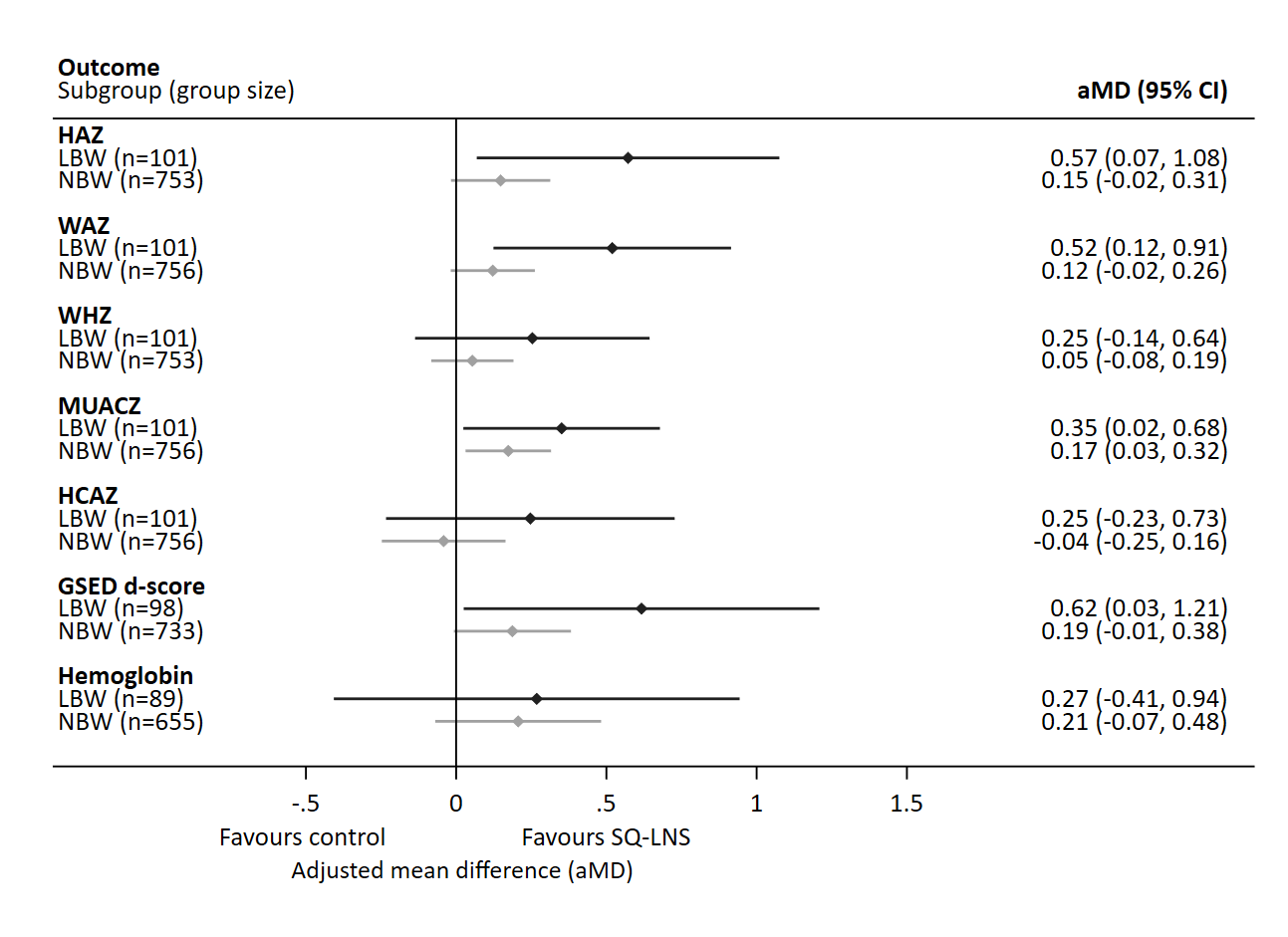 Effect of small quantity lipid nutrient supplements (SQ-LNS) among children born with low birthweight (LBW; <2500 grams) compared to normal birth weight (NBW; ≥2500 grams) on child anthropometric, developmental, and hemoglobin outcomes. Multivariable regression models adjusted for study district, child age at enrollment, child sex, caregiver age, caregiver education category, household assets quintile. HAZ, length/height for age z-scores; WAZ, weight for age z-scores; WHZ, Weight for height z-scores; MUACZ, mid-upper arm circumference z-scores; HCAZ, head circumference z-scores; GSED d-score, Global Scales of Early Development score.
Effect of small quantity lipid nutrient supplements (SQ-LNS) among children born with low birthweight (LBW; <2500 grams) compared to normal birth weight (NBW; ≥2500 grams) on child anthropometric, developmental, and hemoglobin outcomes. Multivariable regression models adjusted for study district, child age at enrollment, child sex, caregiver age, caregiver education category, household assets quintile. HAZ, length/height for age z-scores; WAZ, weight for age z-scores; WHZ, Weight for height z-scores; MUACZ, mid-upper arm circumference z-scores; HCAZ, head circumference z-scores; GSED d-score, Global Scales of Early Development score.
