Neonatal Hemodynamics and Cardiovascular Medicine 1
Session: Neonatal Hemodynamics and Cardiovascular Medicine 1
195 - Exposure-Response Relationships for Indomethacin Use in the NICU Exists for Renal Toxicity and not Efficacy: Insights for Model-Informed Precision Dosing
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 195.4839
Cindy Hoi Ting Yeung, The Hospital for Sick Children, Toronto, ON, Canada; Deanna C. Sekulich, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States; Allison Scott, Children's Mercy Hospitals and Clinics, Blue Springs, MO, United States; Rachel Su, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States; Mhd Wael Alrifai, Vanderbilt University School of Medicine, Nashville, TN, United States; Susan M Lopata, Nationwide Children's Hospital, Columbus, OH, United States; John Jeffrey Reese, Vanderbilt University School of Medicine, Nashville, TN, United States; Anil Maharaj, The University of British Columbia, Vancouver, BC, Canada; Tamorah R. Lewis, The Hospital for Sick Children, Toronto, ON, Canada

Tamorah R. Lewis, MD, PhD (she/her/hers)
Associate Professor
The Hospital for Sick Children
Toronto, Ontario, Canada
Presenting Author(s)
Background: Indomethacin (IND) is commonly used in the Neonatal Intensive Care Unit (NICU) and yet unpredictable clinical efficacy and toxicity occurs with standard weight-based dosing. Under-dosing can lead to ineffective intraventricular hemorrhage (IVH) prevention, and heart surgery for patent ductus arteriosus (PDA). Overdosing can lead to kidney injury and bowel perforation. Model-informed precision dosing (MIPD) uses prior knowledge about patient characteristics and drug pharmacokinetics (PK) to provide individualized doses to overcome deficiencies of standard dosing.
Objective: To identify an IND therapeutic window for MIPD to target, exposure-response relationships (ERR) need to be determined.
Design/Methods: IND PK and demographic data were prospectively and retrospectively collected from preterm infants treated at Vanderbilt University Medical Center (VUMC) and Children’s Mercy Hospital (CMH), respectively. The following ERR were assessed: (1) area under the curve 0-∞ (AUC0-∞) and IVH efficacy (non-severe vs severe), (2) AUC (start to end of course +12 hrs) and PDA efficacy (success vs failure), and (3) peak concentration (Cmax) and renal toxicity (urine output nadir within 12 hours after dose (U/O)). A previously developed IND population PK (popPK) model was modified to predict AUC0-∞ for both cohorts, while the unmodified model was used to predict AUC and Cmax for the CMH cohort (Figure 1). For the ERR analyses, fixed-effect or mixed-effect linear and logistic regression models were developed and implemented in R (R Core Team, 2019, Vienna, Austria).
Results: Eighty-three neonates (1,695 IND concentrations; and 1,820 U/O) were available for analysis. The regression analyses supported a lack of ERR for IVH and PDA efficacy (Figure 2), with only GA as the significant predictor of IVH severity (p = 0.04). A regression model of Cmax, difference of fluid intake 24 hours (mL/kg) after dose from the population median, and subject as a random effect significantly predicted ln U/O. The model was used to simulate U/O < 0.5 and < 1 mL/kg/h for renal toxicity and showed that on average, these levels could be reached with Cmax of 14 µg/mL and 22 µg/mL, respectively (Figure 3).
Conclusion(s): The lack of ERR for IND efficacy may indicate that current dosing does not give exposures sufficiently high to observe an ERR, and thus dose-ranging studies beyond the traditional doses used in this study are needed to demonstrate ERR for a therapeutic window. This target will also need to account for our identified upper exposure limit for renal toxicity.
Figure 1: An overview of the study workflow
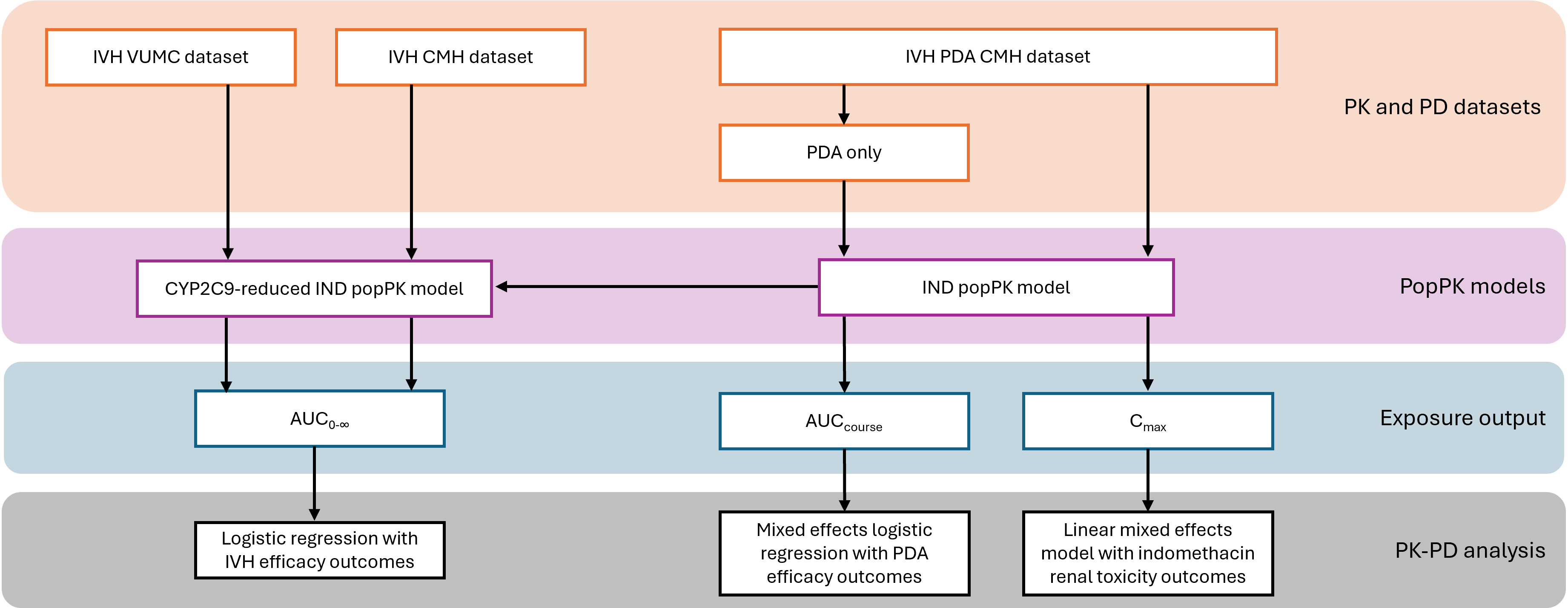 The descriptions of the main steps are presented on the right within their color-coded boxes from orange to grey. The vertical arrows represent steps for producing patient-specific PK parameters and subsequent PK-PD analyses. The horizontal arrow represents the model modification of the published IND popPK model to develop a CYP2C9-reduced IND popPK model. IVH: intraventricular hemorrhage; PDA: patent ductus arteriosus; VUMC: Vanderbilt University Medical Center; CMH: Children’s Mercy Hospital; AUC: area under the curve; IND: indomethacin; popPK: population pharmacokinetics; PK: pharmacokinetics; PD: pharmacodynamics.
The descriptions of the main steps are presented on the right within their color-coded boxes from orange to grey. The vertical arrows represent steps for producing patient-specific PK parameters and subsequent PK-PD analyses. The horizontal arrow represents the model modification of the published IND popPK model to develop a CYP2C9-reduced IND popPK model. IVH: intraventricular hemorrhage; PDA: patent ductus arteriosus; VUMC: Vanderbilt University Medical Center; CMH: Children’s Mercy Hospital; AUC: area under the curve; IND: indomethacin; popPK: population pharmacokinetics; PK: pharmacokinetics; PD: pharmacodynamics. Figure 2: Exposure and Efficacy relationships for IVH and PDA outcomes
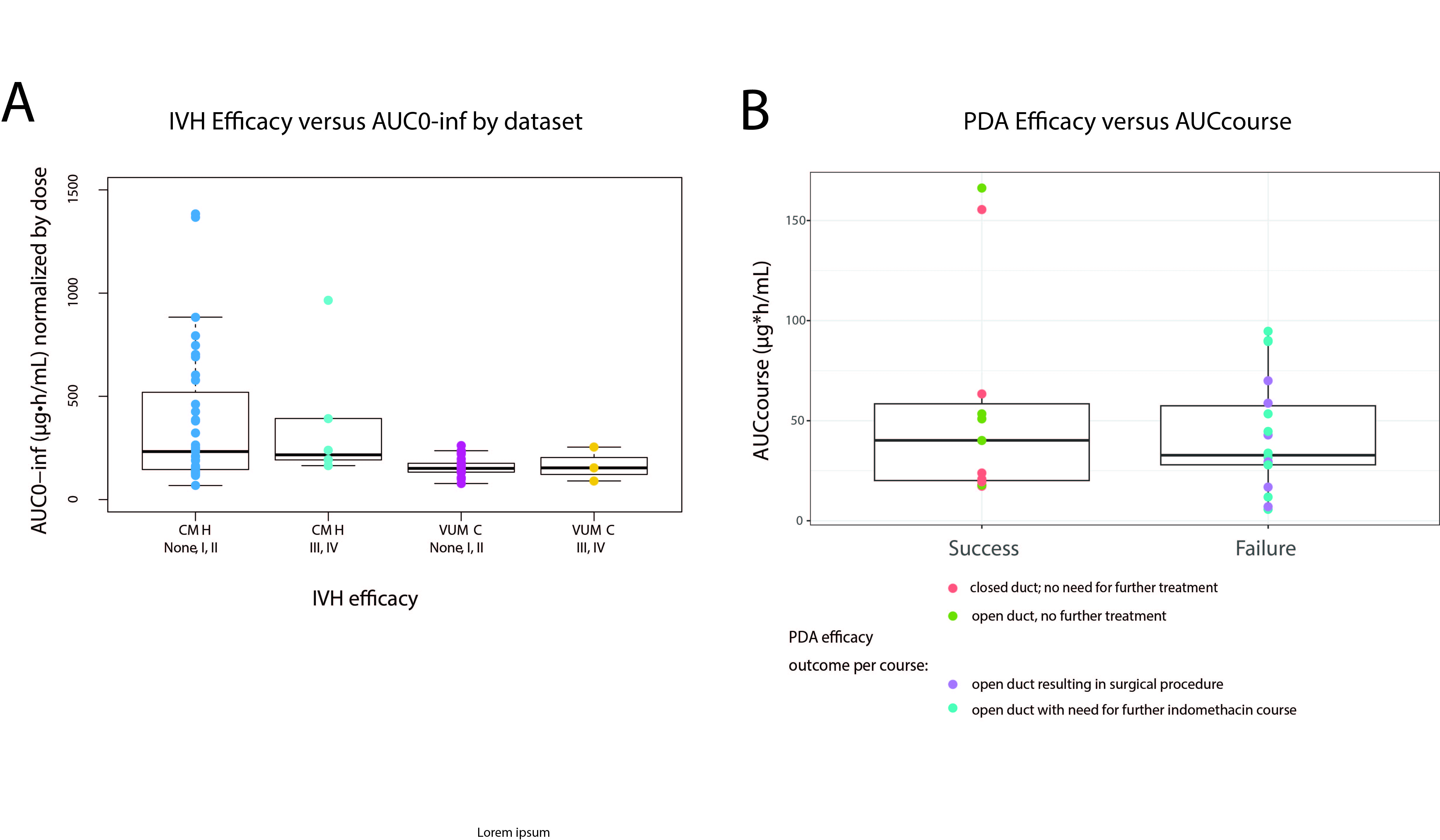 A) AUC0-∞ across intraventricular hemorrhage (IVH) efficacy outcome by severity: non-severe (none, grade I, grade II) and severe (grade III, grade IV), shown per study site (CMH: Children’s Mercy Hospital and VUMC: Vanderbilt University Medical Center). B) AUCcourse across patent ductus arteriosus (PDA) efficacy outcome by severity: success (no further treatment needed) and failure (further indomethacin dose or surgical procedure needed).
A) AUC0-∞ across intraventricular hemorrhage (IVH) efficacy outcome by severity: non-severe (none, grade I, grade II) and severe (grade III, grade IV), shown per study site (CMH: Children’s Mercy Hospital and VUMC: Vanderbilt University Medical Center). B) AUCcourse across patent ductus arteriosus (PDA) efficacy outcome by severity: success (no further treatment needed) and failure (further indomethacin dose or surgical procedure needed).Figure 3: Slated indomethacin-associated renal toxicity
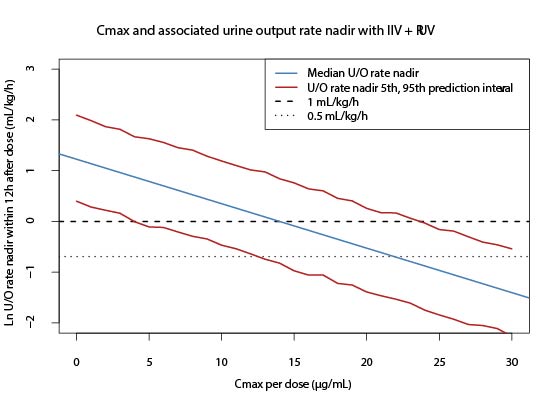 Cmax and associated ln of U/O rate nadir within 12 hours after dose with simulated median, and 5th and 95th prediction interval U/O rate nadir within 12 hours after dose. Both interindividual variability (IIV) and residual unexplained variability (RUV) were used in the simulation. Extrapolation of data was extended past U/O rate nadir within 12 hours after dose <0.5 mL/kg/h and <1 mL/kg/h.
Cmax and associated ln of U/O rate nadir within 12 hours after dose with simulated median, and 5th and 95th prediction interval U/O rate nadir within 12 hours after dose. Both interindividual variability (IIV) and residual unexplained variability (RUV) were used in the simulation. Extrapolation of data was extended past U/O rate nadir within 12 hours after dose <0.5 mL/kg/h and <1 mL/kg/h.
