Quality Improvement/Patient Safety 2
Session: Quality Improvement/Patient Safety 2
024 - Improving Antiviral Use in Children Hospitalized with Influenza
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 24.5214
Sabrina E. Carro, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States; James W. Antoon, Vanderbilt University Medical Center, Nashville, TN, United States; Mhd Wael Alrifai, Vanderbilt University School of Medicine, Nashville, TN, United States; David P. Johnson, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States

Sabrina E. Carro, MD (she/her/hers)
Fellow
Monroe Carell Jr. Children's Hospital at Vanderbilt
Nashville, Tennessee, United States
Presenting Author(s)
Background: In the United States, seasonal influenza infections result in about 25,000 children hospitalized annually. The Centers for Disease Control, Infectious Diseases Society of America, and American Academy of Pediatrics recommend antiviral treatment in all children hospitalized with influenza. Adherence to national guidelines remains suboptimal.
Objective: To increase influenza-specific antiviral use in hospitalized children diagnosed with influenza who are discharged from the Pediatric Hospital Medicine (PHM) Service from 74.8% to >90% in two influenza seasons.
Design/Methods: A multi-disciplinary team utilized quality improvement methodology to create a key driver diagram (Figure 1). Our population included all encounters discharged from the PHM service with a positive influenza respiratory test within 5 days of admission or influenza diagnosis on the hospital problem list. Outcome measure was the percentage of encounters with at least one dose of a hospital formulary antiviral (oseltamivir or peramivir) administered. Process measure was the average time from influenza infection identified to antiviral order signed. Balancing measure was the number of encounters between events of inappropriate antiviral use, excluding empiric treatment for influenza-like illness. Baseline data included the 2022-2023 influenza season through the first intervention. Once interventions began, data were followed prospectively via a real time dashboard and grouped in 12 consecutive encounters for the outcome measure. Interventions included dynamic, conditional oseltamivir orders in the admission order set, a clinical decision support alert, and education efforts. Statistical process control charts were used to analyze all measures.
Results: Baseline consisted of 143 encounters with antiviral treatment administered in 74.8% of encounters. There has not been a shift in the center line in the outcome measure, though 7 of last 8 points are above centerline (Figure 2). Special cause variation (SCV) was seen in our process measure with a shift in the center line from 4.9 hours to 2.4 hours following educational interventions (Figure 3). Balancing measure revealed no special cause variation (not shown).
Conclusion(s): Our most recent outcome measure results are encouraging with 7 of 8 points above the centerline, though SCV has not been achieved yet. Data tracking continues to help determine if higher reliability interventions such as those in the electronic health record will result in a system change indicating increased appropriate antiviral administration as this influenza season begins.
Figure 1: Key Driver Diagram
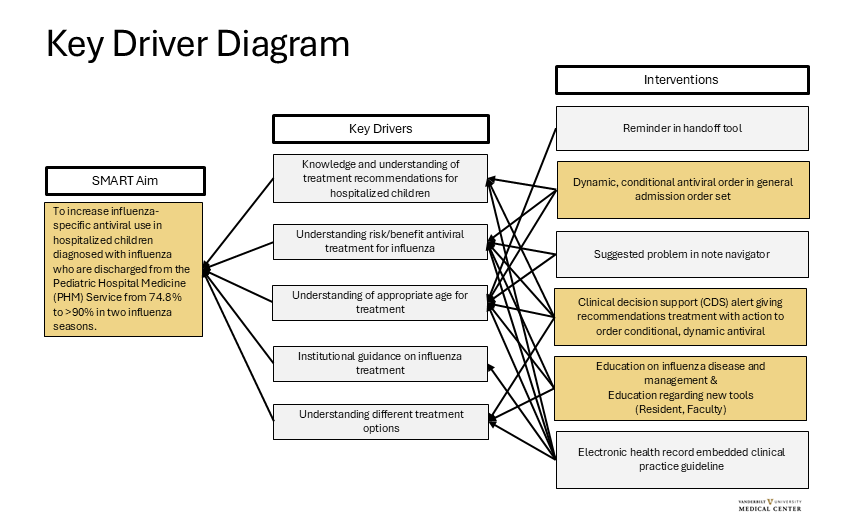 A Key Driver Diagram was created with a multi-disciplinary team including: pediatric hospital medicine, pediatric infectious diseases, pharmacy, and clinical informatics. Children are diagnosed with influenza by respiratory testing either at our institution or at an outside institution within 5 days of admission. Interventions in gold shaded boxes are in progress. The dynamic, conditional orders uses logic to display an oseltamivir order with the appropriate age and weight-based dose during the admission workflow. If respiratory testing comes back after that workflow, a delayed alert will display prompting the clinician to order an antiviral. There is logic to suppress the alert if an antiviral is ordered or if one dose has been successfully administered. In the alert, the clinician is encouraged to discuss this recommendation with family if not already addressed. Additionally, the alert can be dismissed for the rest of the encounter if the family prefers to not treat with an antiviral. Interventions in grey shaded boxes are potential future interventions.
A Key Driver Diagram was created with a multi-disciplinary team including: pediatric hospital medicine, pediatric infectious diseases, pharmacy, and clinical informatics. Children are diagnosed with influenza by respiratory testing either at our institution or at an outside institution within 5 days of admission. Interventions in gold shaded boxes are in progress. The dynamic, conditional orders uses logic to display an oseltamivir order with the appropriate age and weight-based dose during the admission workflow. If respiratory testing comes back after that workflow, a delayed alert will display prompting the clinician to order an antiviral. There is logic to suppress the alert if an antiviral is ordered or if one dose has been successfully administered. In the alert, the clinician is encouraged to discuss this recommendation with family if not already addressed. Additionally, the alert can be dismissed for the rest of the encounter if the family prefers to not treat with an antiviral. Interventions in grey shaded boxes are potential future interventions. Figure 2: Outcome Measure
.png) P-Chart of hospital admission encounters of children diagnosed with influenza who were discharged from the Pediatric Hospital Medicine (PHM) service that had at least one successful administration of an influenza-specific antiviral. The first intervention was a hospital-wide Grand Rounds. Other educational interventions were targeted to either PHM faculty or residents. For the dynamic order and CDS alert interventions, there was resident education on the new tool as part of standard change management best practices. Baseline: 74.8%, Goal Line: 90%. Abbreviation: CDS: Clinical Decision Support.
P-Chart of hospital admission encounters of children diagnosed with influenza who were discharged from the Pediatric Hospital Medicine (PHM) service that had at least one successful administration of an influenza-specific antiviral. The first intervention was a hospital-wide Grand Rounds. Other educational interventions were targeted to either PHM faculty or residents. For the dynamic order and CDS alert interventions, there was resident education on the new tool as part of standard change management best practices. Baseline: 74.8%, Goal Line: 90%. Abbreviation: CDS: Clinical Decision Support.Figure 3: Process Measure
.png) X-bar chart with average time to an antiviral medication order signed by a clinician. If a respiratory test was obtained during the encounter, the time from the test result to order signed was used. If the test was prior to the current encounter, the time from presentation to the emergency department to order signed was used. There was special cause variation with 8 points below the mean causing a shift in the center line from 4.9 hours to 2.4 hours after educational interventions. There was also special cause variation with one point outside the upper control limit at points (1), (2), and (3). Each implicated encounter underwent manual chart review. In (1), there was a delay in an antiviral medication order signed in two of the three encounters for that data point. The first was due to escalating respiratory support with inability to obtain enteral access via a nasogastric tube and the second was due to the patient having repeated emesis resulting in an order delay and successful administration. At (2), the family preferred to discuss further before proceeding with treatment which led to the delay. At (3), there was a miscommunication during care handoffs as to whether the patient completed an oseltamivir treatment course outpatient prior to admission.
X-bar chart with average time to an antiviral medication order signed by a clinician. If a respiratory test was obtained during the encounter, the time from the test result to order signed was used. If the test was prior to the current encounter, the time from presentation to the emergency department to order signed was used. There was special cause variation with 8 points below the mean causing a shift in the center line from 4.9 hours to 2.4 hours after educational interventions. There was also special cause variation with one point outside the upper control limit at points (1), (2), and (3). Each implicated encounter underwent manual chart review. In (1), there was a delay in an antiviral medication order signed in two of the three encounters for that data point. The first was due to escalating respiratory support with inability to obtain enteral access via a nasogastric tube and the second was due to the patient having repeated emesis resulting in an order delay and successful administration. At (2), the family preferred to discuss further before proceeding with treatment which led to the delay. At (3), there was a miscommunication during care handoffs as to whether the patient completed an oseltamivir treatment course outpatient prior to admission.

