Immunizations/Delivery 2
Session: Immunizations/Delivery 2
685 - Human in vitro modeling determines the immunogenicity of vaccine adjuvant formulations in children
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 685.5365
Sanya Thomas, Boston Children's Hospital, Boston, MA, United States; Caitlin Syphurs, Boston Children's Hospital, Boston, MA, United States; Kevin M. Ryff, Boston Children's Hospital, Cambridge, MA, United States; Jee Hae Han, Boston Children's Hospital, Brookline, MA, United States; Kayla Lesch, Boston Children's Hospital, Boston, MA, United States; Nicole Heredia, Boston Children's Hospital, Boston, MA, United States; Sophie Baden, Boston Children's Hospital, boston, MA, United States; Zachary Ready Miller, Boston Children's Hospital, Cambridge, MA, United States; Ofer Levy, Precision Vaccines Program, Boston Children's Hospital, Boston, MA, United States; Joann Diray-Arce, Harvard Medical School, Boston, MA, United States; Simon D.. van Haren, Boston Children's Hospital, Boston, MA, United States
- ST
Sanya Thomas, MD, MPH, MMSc
Research Fellow
Boston Children's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Infections cause significant morbidity and mortality, particularly among vulnerable populations such as children, making vaccination an effective tool for mitigating this burden.
Objective: One strategy to overcome the challenge of weak and short-lived immunity is to include adjuvants in vaccines to induce a stronger and more durable antigen-specific response.
Design/Methods: To better understand vaccine-induced responses in an age-specific setting, we employed human in vitro models to evaluate the immunogenic potential of primary human leukocytes in response to adjuvant formulations (liposomal and oil-in-water formulations of MPLA, QS-21, MPLA+QS-21; unformulated MPLA, QS-21, QS-7, MPLA+QS-21; dmLT; squalene oil-in-water emulsion; 3M-052 absorbed to alum; and 3M-052 in oil-in-water). We obtained up to 20 mL of peripheral blood per participant from a cohort of healthy children aged 2-6 years to test how children respond to adjuvant stimulation compared to young adults aged 18-45 years. Baseline cellular immune memory status to influenza HA, RSV, and SARS-CoV-2 antigens was assessed using an activation-induced marker assay.
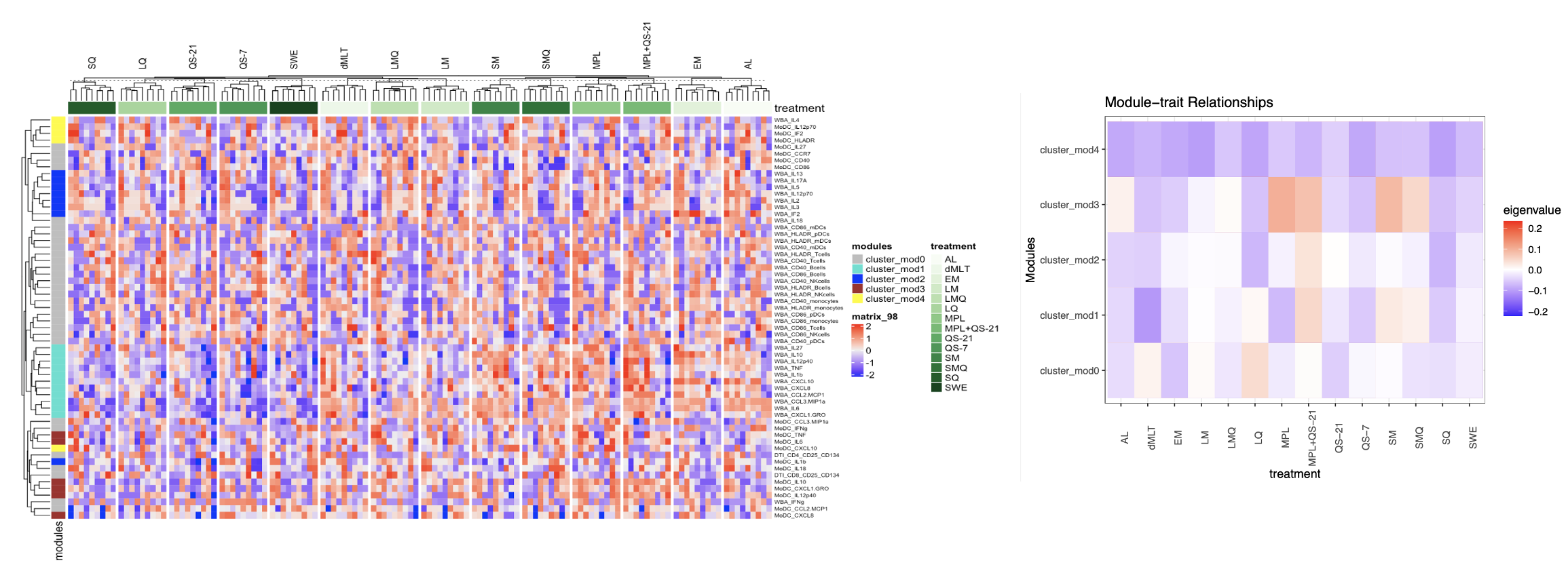
Results: Children demonstrated a greater degree of variation in the magnitude and phenotype of cellular immune memory to RSV (due to exposure), and influenza and SARS-CoV-2 (due to immunization and/or exposure) than adults. In vitro modeling of innate and adaptive immune responses to adjuvant stimulation using combined whole blood assay (WBA), dendritic cell assay (DCA), and antigen-specific T-cell assay (TCA) platforms in these children revealed cross-platform differences in adjuvant mode of action and divergent functional immune responses compared to adults. Unsupervised hierarchical clustering via Weighted Gene Co-expression Network Analysis identified co-expression of pro-inflammatory cytokines and chemokines (IFNg, IL-18, IL-27, CCL2, and CCL3) and activation markers (CD40, CD86, HLA-DR, CCR7, CD25, and CD134) in WBA, DCA, and TCA with higher expression levels associated with unformulated MPLA+QS-21, unformulated QS-21, unformulated MPLA, unformulated QS-7, 3M-052 in oil-in-water, liposomal formulation of QS-21, and dmLT in children.
Conclusion(s): The degree of heterogeneity in pre-existing immunity to viral antigens and the identification of adjuvant formulations able to induce potent inflammatory and antigen-specific cellular responses in pediatric leukocytes may define the distinct mechanisms underlying immune activation in children post-immunization and inform development of adjuvanted pediatric vaccines that induce effective and durable immunity against respiratory viruses.
Children demonstrate greater degree of heterogeneity in terms of frequency of antigen-specific T cells
.png)
Co-expression of pro-inflammatory cytokines and chemokines, and activation markers, and their association with adjuvant formulations in children