Allergy, Immunology, and Rheumatology 1
Session: Allergy, Immunology, and Rheumatology 1
642 - Long-Term Outcomes of Patients with Pediatric Systemic Lupus Erythematosus (pSLE) Treated with Combination Rituximab and Cyclophosphamide: A Single Center Cohort and Review of Literature
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 642.5869
Peyton Watkins, Stony Brook Children's Hospital, Miller Place, NY, United States; Aneesha Achar, Renaissance School of Medicine at Stony Brook University, Clifton Park, NY, United States; Julie Cherian, Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, United States; Farzana Nuruzzaman, Stony Brook Children's Hospital, Stony Brook, NY, United States

Peyton Watkins, Doctor of Medicine (she/her/hers)
Resident PGY-3
Stony Brook Children's Hospital
Miller Place, New York, United States
Presenting Author(s)
Background: Patients with pSLE with life/organ threatening manifestations are treated with a protocol of rituximab and cyclophosphamide at our institution as previously described [1]. However, the few published reports of the combined rituximab and cyclophosphamide in pSLE lacked representation of Black patients and/or evaluation of long-term damage indices beyond 1 year.
Objective: To evaluate long-term Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores, exposure to steroids, and Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Indices of patients with pSLE treated with a protocol of rituximab and cyclophosphamide and review its use in the literature.
Design/Methods: Retrospective review of medical records between 2013-2023 of patients diagnosed with SLE at ≤18 years old and treated with rituximab and cyclophosphamide with at least 1 year of follow-up were found. Descriptive statistics were calculated with continuous variables as means and categorical variables as percentages. A PubMed search for articles published after 2000 was performed using search terms “cyclophosphamide and rituximab” AND “systemic lupus erythematosus” AND “pediatric patients OR children OR adolescents” to compare prior studies with our cohort.
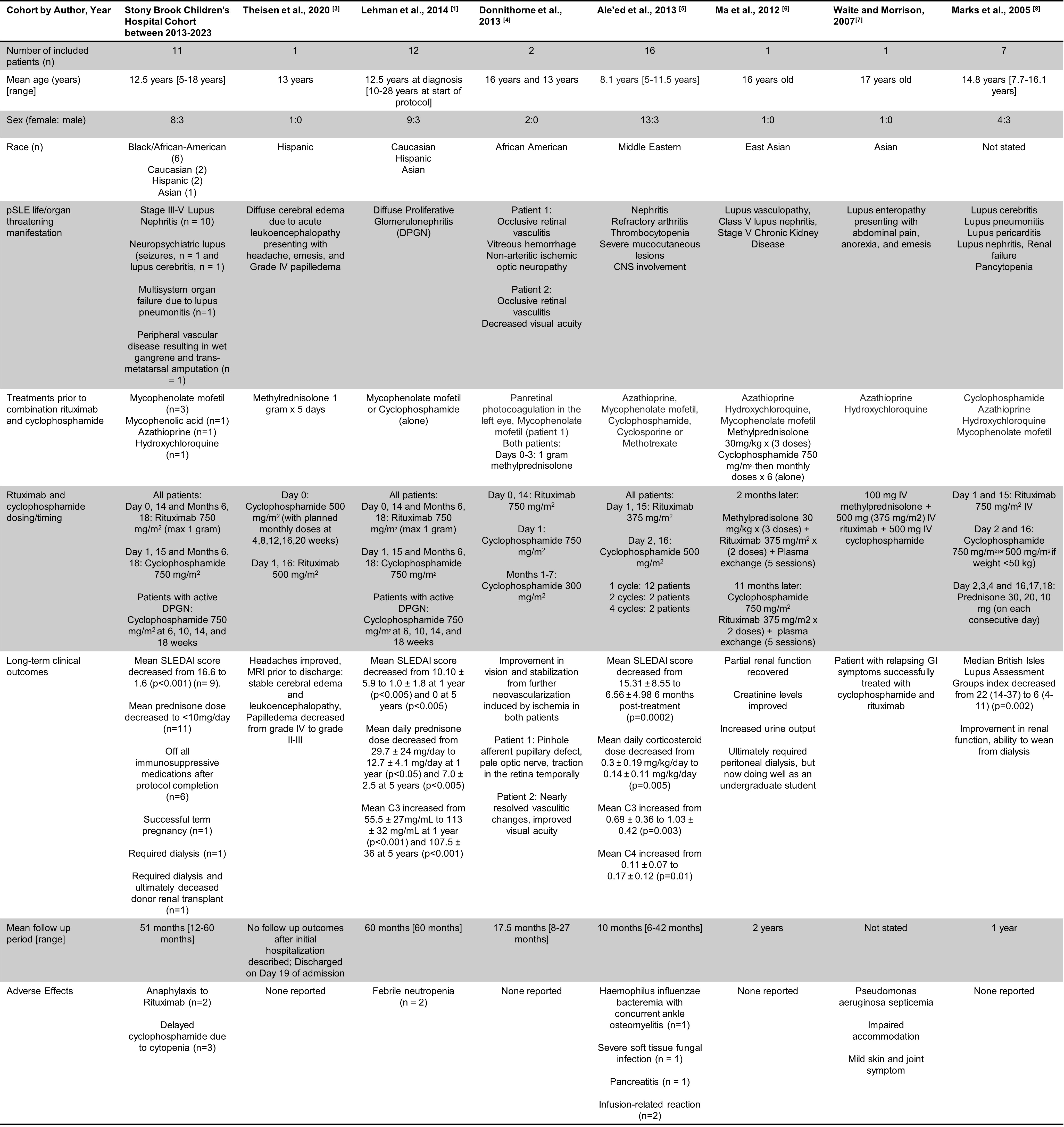
Results: Eleven patients met eligibility criteria (Table 1). Mean prednisone dose and mean SLEDAI scores decreased significantly over follow-up periods. We achieved low disease activity in all our patients as defined by SLEDAI score < 4 and prednisone dose < 7.5 mg/day (Figure 1). There was no statistically significant difference between SLICC/ACR damage indices over time. No difference in outcomes between races was seen. Seven studies from case reports (n=1) to small cohorts (n= 17) were included in our literature review (Table 2). Our cohort had a more diverse population and included longer-term mean outcomes than earlier studies (51 months versus 18 months, respectively). Despite some variability in dosing, all studies reported improvement of disease activity and relatively low incidence of infections.
Conclusion(s): Our racially diverse cohort shows the efficacy of systematic administration of rituximab and cyclophosphamide in decreasing disease activity scores and decreasing overall exposure to steroids in pSLE. Long-term organ damage was not seen 5 years after treatment in our cohort, further emphasizing disease control with early aggressive therapy. This protocol was well-tolerated. Larger, prospective randomized clinical trials are needed for further validation.
Table 1: Demographics and clinical characteristics of pSLE patients treated with Rituxumab/Cyclophosphamide protocol at Stony Brook Children's
Figure 1a-1c
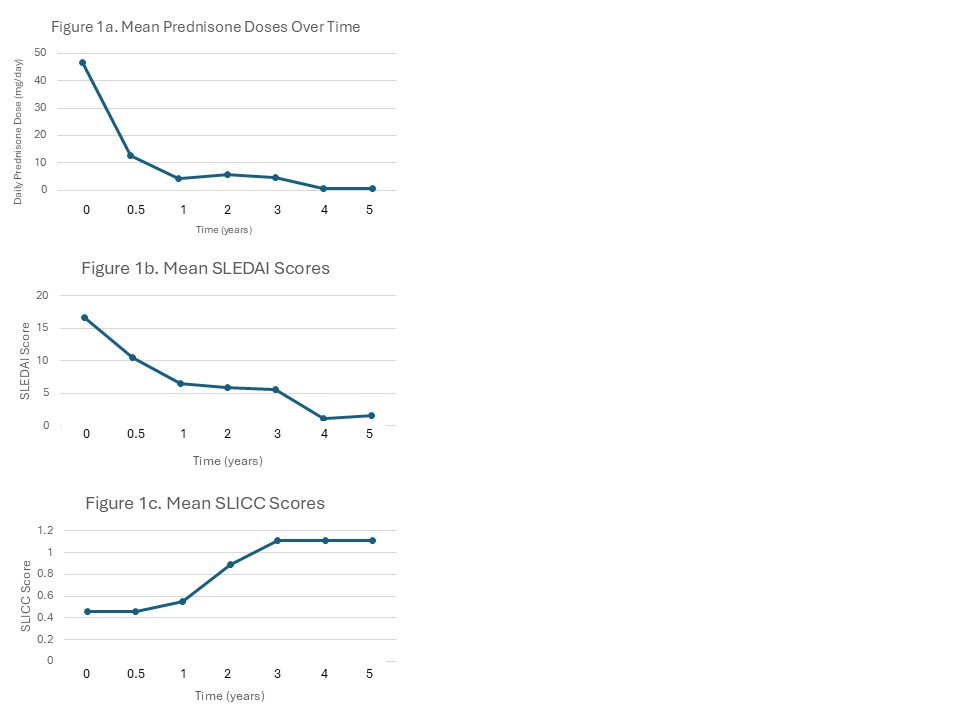 Mean prednisone dosage decreased significantly from initiation of protocol to 5 years post-treatment from 46.8 mg/day to 0.6 mg/day (p-value 0.002) (Figure 1a). Mean SLEDAI scores also significantly decreased from 16.6 to 1.6 (p-value < 0.001) (Figure 1b). Thus, we achieved low disease activity as defined by SLEDAI-2K score less than 4 and prednisone dose less than 7.5 mg/day with this protocol. There was no statistically significant difference between SLICC/ACR damage indices at the start of the Cyclophosphamide and Rituximab protocol and at five years follow up (0.5 to 1.1, p-value 0.14) (Figure 1c).
Mean prednisone dosage decreased significantly from initiation of protocol to 5 years post-treatment from 46.8 mg/day to 0.6 mg/day (p-value 0.002) (Figure 1a). Mean SLEDAI scores also significantly decreased from 16.6 to 1.6 (p-value < 0.001) (Figure 1b). Thus, we achieved low disease activity as defined by SLEDAI-2K score less than 4 and prednisone dose less than 7.5 mg/day with this protocol. There was no statistically significant difference between SLICC/ACR damage indices at the start of the Cyclophosphamide and Rituximab protocol and at five years follow up (0.5 to 1.1, p-value 0.14) (Figure 1c).Table 2: Comparison of patients with pSLE treated with Rituxumab/Cyclophosphamide protocol at Stony Brook Children's to patients with pSLE treated with Rituximab/Cyclophosphamide in previous literature