Neonatal Neurology 3
Session: Neonatal Neurology 3
369 - Dexmedetomidine Exposure-Response Relationships in Neonates with HIE Undergoing Therapeutic Hypothermia
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 369.4420
Athanasios Chamzas, Center for Translational Medicine, University of Maryland Baltimore, Baltimore, MD, United States; Fulden Aycan, University of Iowa Stead Family Children's Hospital, Iowa, IA, United States; Pilli Nageswara, University of Maryland Baltimore, Baltimore, MD, United States; Kane Maureen, University of Maryland School of Pharmacy, Baltimore, MD, United States; Dina El-Metwally, University of Maryland School of Medicine, Baltimore, MD, United States; Mathangi Gopalakrishnan, University of Maryland, Baltimore, MD, United States

Athanasios Chamzas, MS (he/him/his)
PhD Student
Center for Translational Medicine, University of Maryland Baltimore
Baltimore, Maryland, United States
Presenting Author(s)
Background: Neonates with moderate to severe hypoxic-ischemic encephalopathy (HIE) undergoing therapeutic hypothermia (TH) often receive opioids such as fentanyl and morphine for sedation and shivering prevention. However, opioids can induce hypotension, respiratory depression, and delayed gastrointestinal motility. Dexmedetomidine, an alpha-2 adrenergic agonist, provides sedation and analgesia without compromising respiratory function or gastrointestinal function, making it a promising alternative. Despite increasing off-label use in neonatal care, optimal dosing for effective sedation while minimizing hemodynamic adverse effects is not well established.
Objective: To evaluate the pharmacokinetics (PK) of dexmedetomidine in neonates undergoing TH for HIE and assess the concentration-response relationships for safety and efficacy.
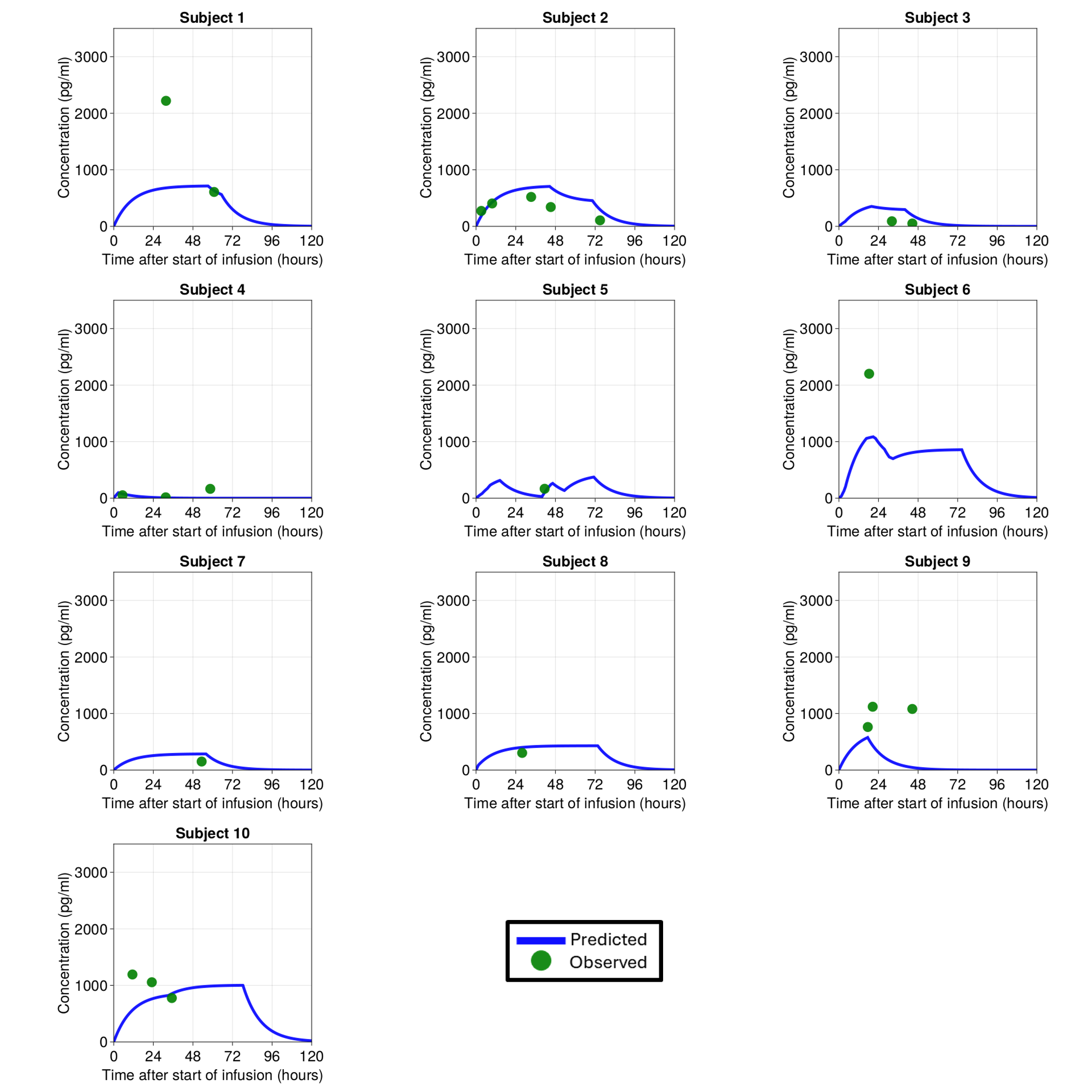
Design/Methods: A prospective pharmacokinetic study was conducted at the University of Maryland Medical Center, including neonates with HIE undergoing TH who received dexmedetomidine as part of routine care. Plasma concentrations were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). A population PK model (McAdams et al,2020) was utilized to predict individual dexmedetomidine concentrations. These were correlated with clinical outcomes—including Neonatal Pain, Agitation, and Sedation Scale (N-PASS) scores and hemodynamic parameters—to develop concentration-response relationships.
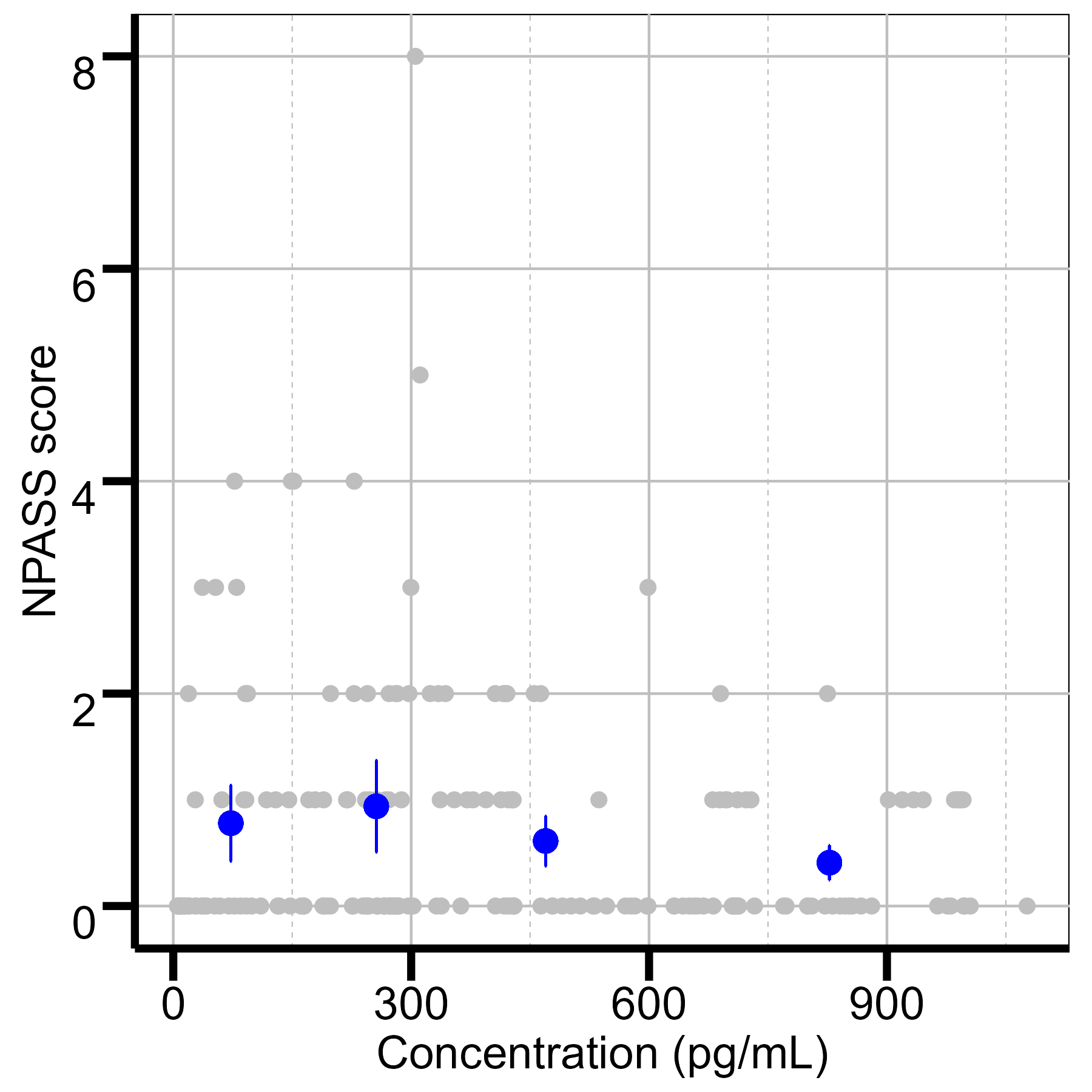
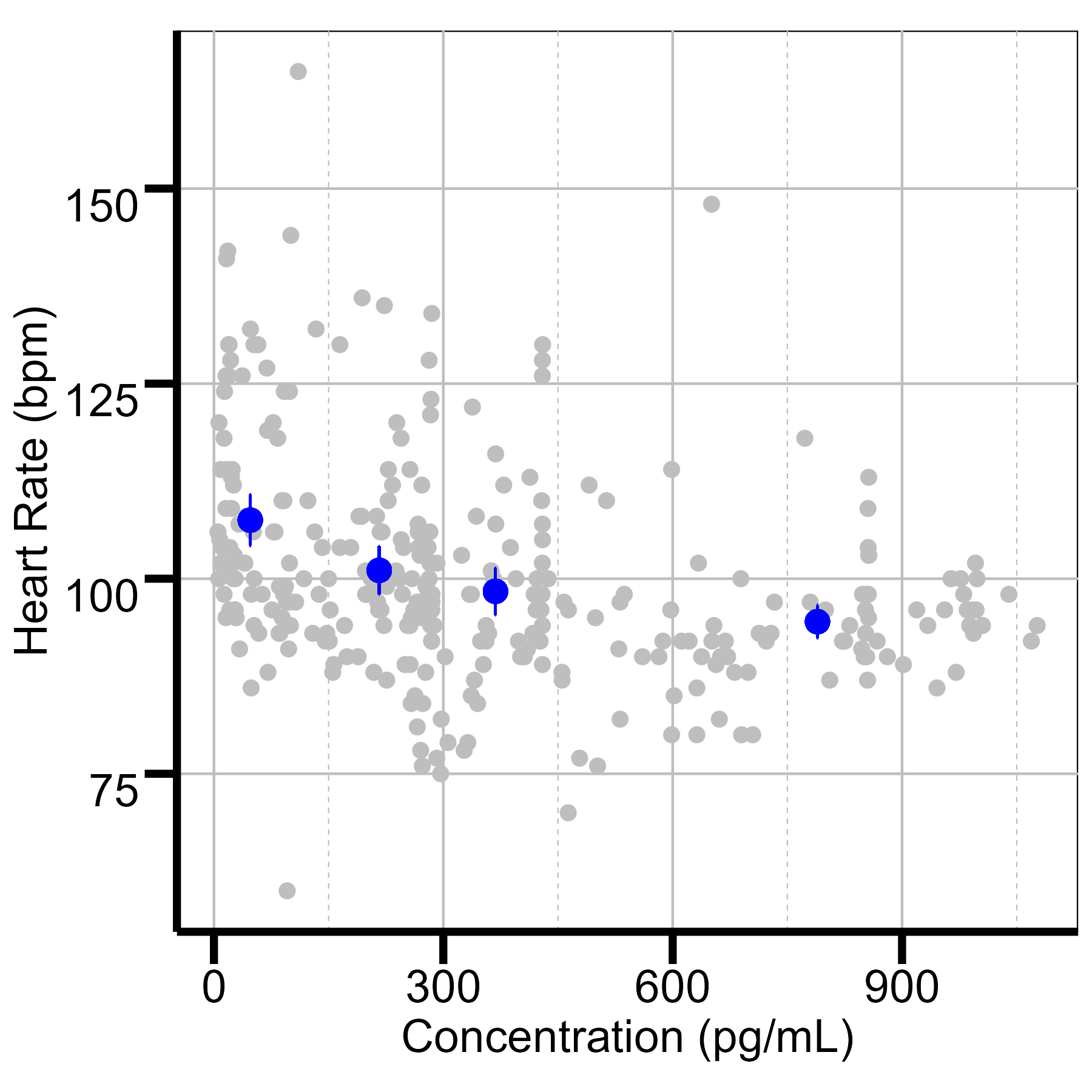
Results: Eleven neonates were included, with an average birthweight of 3.44 ±0.85 kg and gestational age of 38.5 ±1.6 weeks. 23 blood samples were analyzed, with concentrations ranging from 16-2,220 pg/mL at infusion rates of 0.2–1.0 mcg/kg/h. Each neonate contributed an average of two blood samples, with repeated heart rate (median: 46) and N-PASS score (median: 36) measurements. A one-compartment model adequately described the PK of dexmedetomidine. Estimated clearance was 0.85 L/h, consistent with values reported for non-HIE neonates (0.548–1.23 L/h). Higher dexmedetomidine exposure was associated with reduced heart rate and N-PASS scores. Concentrations of 300–350 pg/mL corresponded to heart rates of 80–100 bpm, avoiding bradycardia, while 250–300 pg/mL achieved adequate sedation.
Conclusion(s): This study provides preliminary concentration-response relationships for dexmedetomidine in neonates with HIE undergoing TH. The developed dexmedetomidine concentration-response relationship will be refined with additional data to optimize dosing for effective sedation with minimal adverse effects in this vulnerable population.
Individual concentration time profiles
Quantile plot of heart rate vs concentration
Quantile plot of NPASS score vs concentration