Neonatal Neurology 1
Session: Neonatal Neurology 1
341 - Altered Maturational Trajectory of Cerebral Metabolites in Preterm Infants
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 341.3767
Anola Stage, Children's National Health System, Alexandria, VA, United States; Katherine Ottolini, George Washington University School of Medicine and Health Sciences, District of Columbia, DC, United States; Steve CN. Hui, Children's National Health System, Washington, DC, United States; Julius Ngwa, Children's Hospital, Washington, DC, United States; Sudeepta Basu, Children's National Health System, Washington, DC, United States; Kushal Kapse, Children's National Health System, Washington, DC, United States; Aaron Zeman, Children's National Health System, Washington, DC, United States; Nickie Andescavage, Children's National Health System, Washington, DC, United States; Catherine Limperopoulos, Children's National Health System, Washington DC, DC, United States

Anola Stage, MD, IBCLC (she/her/hers)
Fellow
Children's National Health System
Alexandria, Virginia, United States
Presenting Author(s)
Background: The third trimester of pregnancy is a critical period of rapid fetal brain growth and maturation. However, it remains unclear whether current neonatal intensive care practices, following preterm delivery, support optimal biochemical development in the preterm neonatal brain as in-utero. Magnetic resonance spectroscopy (MRS) provides a non-invasive method to evaluate brain metabolites including N-Acetylaspartate (NAA), Creatine (Cr) and myo-Inositol (mI). These metabolites play unique roles during brain maturation; NAA is a marker of neuronal activity, Cr serves as a marker of cellular energy, while mI is a key component to cellular growth.
Objective: The aim of this study was to compare cerebral metabolite development across the second and third trimester between in-utero healthy fetuses and ex-utero preterm infants.
Design/Methods: Patients were prospectively enrolled. Inclusion criteria for healthy fetuses comprised singleton gestation in the second and third trimester. Preterm neonates were eligible if delivered < 37 weeks of gestational age (GA) without evidence of moderate-severe brain injury using Kidokoro score ( < 8). All participants underwent 1.5T brain MRI and MRS (TR=1500; TE=35 ms) with voxels for fetuses and preterm neonates located in the mid-cerebrum and frontal white matter, respectively (Figure 1). Metabolite levels were reported in water-scaled absolute measurements. A total of 8 cerebral metabolites were analyzed using a random intercept linear mixed effects model adjusting for age (gestational or postmenstrual) at the time of the scan.
Results: MRS data were acquired for 140 subjects (82 fetus, 59 preterm), with age at MRI scan ranging from 23.0 to 41.4 weeks (preterm 33.0 +/- 5.7, fetus 33.3 +/- 4.8) (Table 1). Compared to healthy fetuses, preterm infants demonstrated a declining trajectory of total N-Acetylaspartate (tNAA) (β = -0.362, p = 0.009) and total creatine (tCr) (β = -0.341, p = 0.002) levels while myo-inositol (mI) (β = 0.692, p = 0.007) levels rose across the second and third trimester (Figure 2a, b, c).
Conclusion(s): Preterm infants developing in extrauterine environment demonstrated altered brain biochemical development. The observed alterations of metabolite trajectories in preterm neonates underscores the concern that the current intensive care practices may not be adequate to support the normal biochemical maturation of the brain after preterm birth.
Table 1.
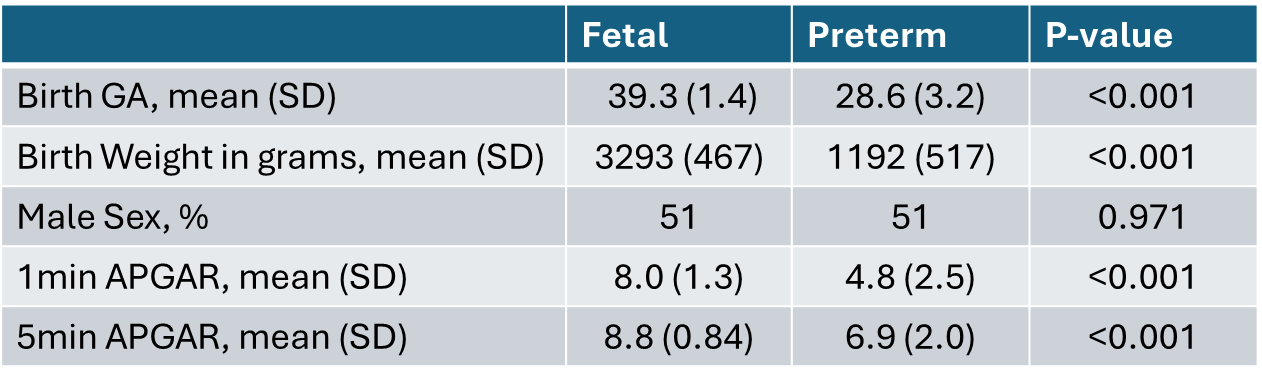 Characteristics of fetal and neonatal subjects.
Characteristics of fetal and neonatal subjects.Figure 1.
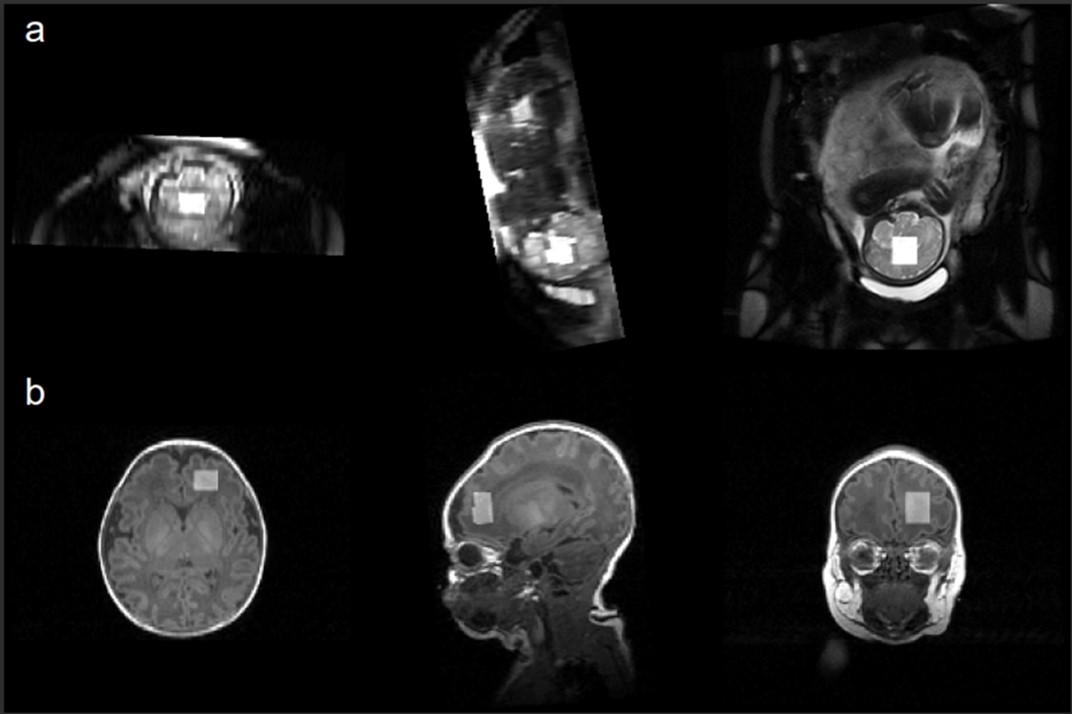 Voxel placement in the mid-cerebrum and frontal white matter for fetal (a) and neonatal (b) MRI, respectively.
Voxel placement in the mid-cerebrum and frontal white matter for fetal (a) and neonatal (b) MRI, respectively.Figure 2.
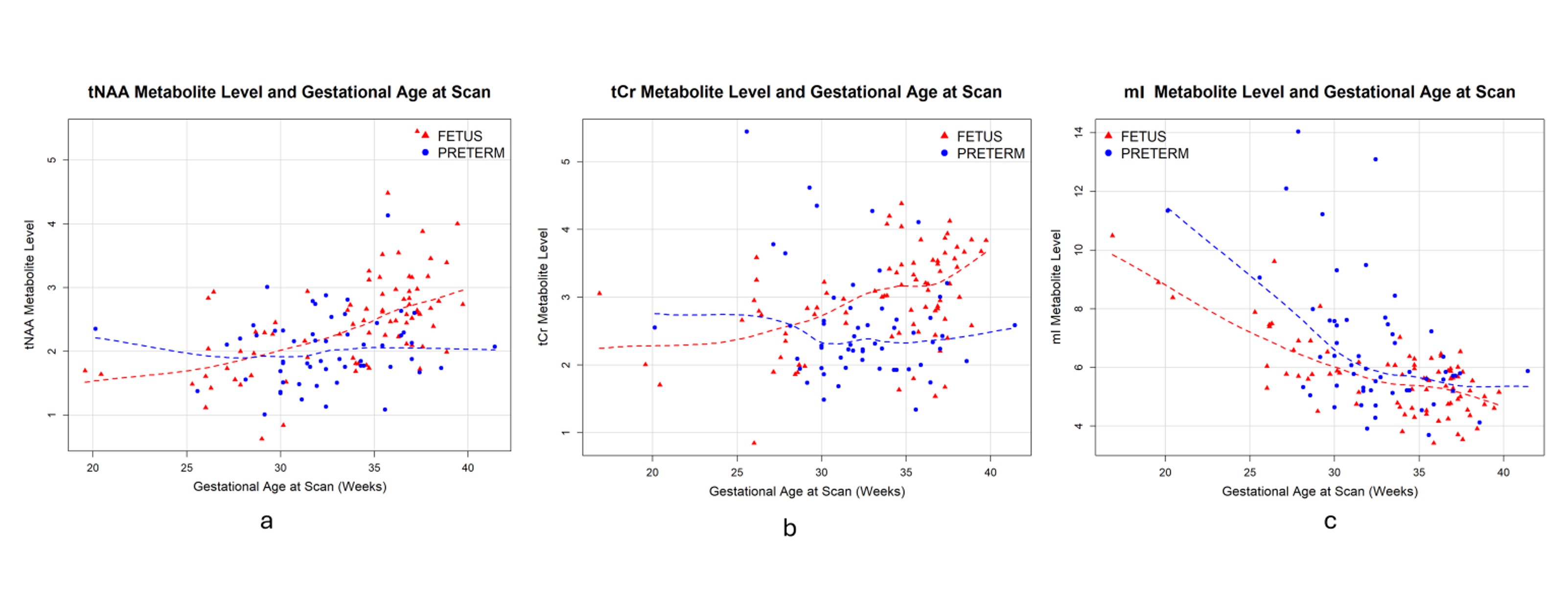 Scatter plots of metabolite levels of total N-Acetylaspartate (tNAA), total Creatine (tCr) and myo-Inositol (mI).
Scatter plots of metabolite levels of total N-Acetylaspartate (tNAA), total Creatine (tCr) and myo-Inositol (mI). 
