Neonatal/Infant Resuscitation 2
Session: Neonatal/Infant Resuscitation 2
308 - Comparison of heart rate performance between two pulse oximeters in newborns
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 308.4198
Manoj Biniwale, Cedars Sinai Medical Center, Los Angeles, CA, United States; Laura Dean, Wake Forest School of Medicine of Wake Forest Baptist Medical Center, Winston Salem, NC, United States; Hui Xiong, Medtronic, Maple Grove, MN, United States; Rangasamy Ramanathan, Cedars Sinai Guerin Children's, Cedars Sinai Medical Center, Los angeles, CA, United States

Manoj Biniwale, MD (he/him/his)
Director of NICU Clinical Research
Cedars Sinai Medical Canter
Los Angeles, California, United States
Presenting Author(s)
Background: Pulse oximetry plays a pivotal role in continuously monitoring blood oxygen saturation (SpO2) and pulse rate (PR). The Neonatal Resuscitation Program® recommends pulse oximetry monitoring for infants requiring respiratory support at birth.
Objective: The primary objective was to evaluate time to pulse oximeter stable signal, compared to reference electrocardiogram (EKG) for newborns in the delivery room.
Design/Methods: This was a prospective, non-randomized, multi-center, pilot study. EKG monitor and two pulse oximeters (Nellcor™ N-600x with Oxysoft™ neonatal-adult SpO2 sensor, Medtronic and Masimo® Rad-97™ with LNCS®-Neo sensor, Masimo®), each set at the highest sensitivity, were simultaneously connected to participants’ feet right after birth. All monitors were connected to a data acquisition system for simultaneous, continuous data collection. Blood gases were not collected. All newborns were observed until a subjective, investigator-designated stable signal, defined as simultaneous SpO2 and PR signals for at least three beats appropriate for clinical appearance, was obtained for at least two minutes. PR accuracy was evaluated for root-mean-square difference between pulse oximeters and EKG heart rate. Bland-Altman plots were generated with linear regression fit, mean, and 95% limits of agreement. A post-hoc, simulation analysis was conducted using raw device data for an objective time to stable signal.
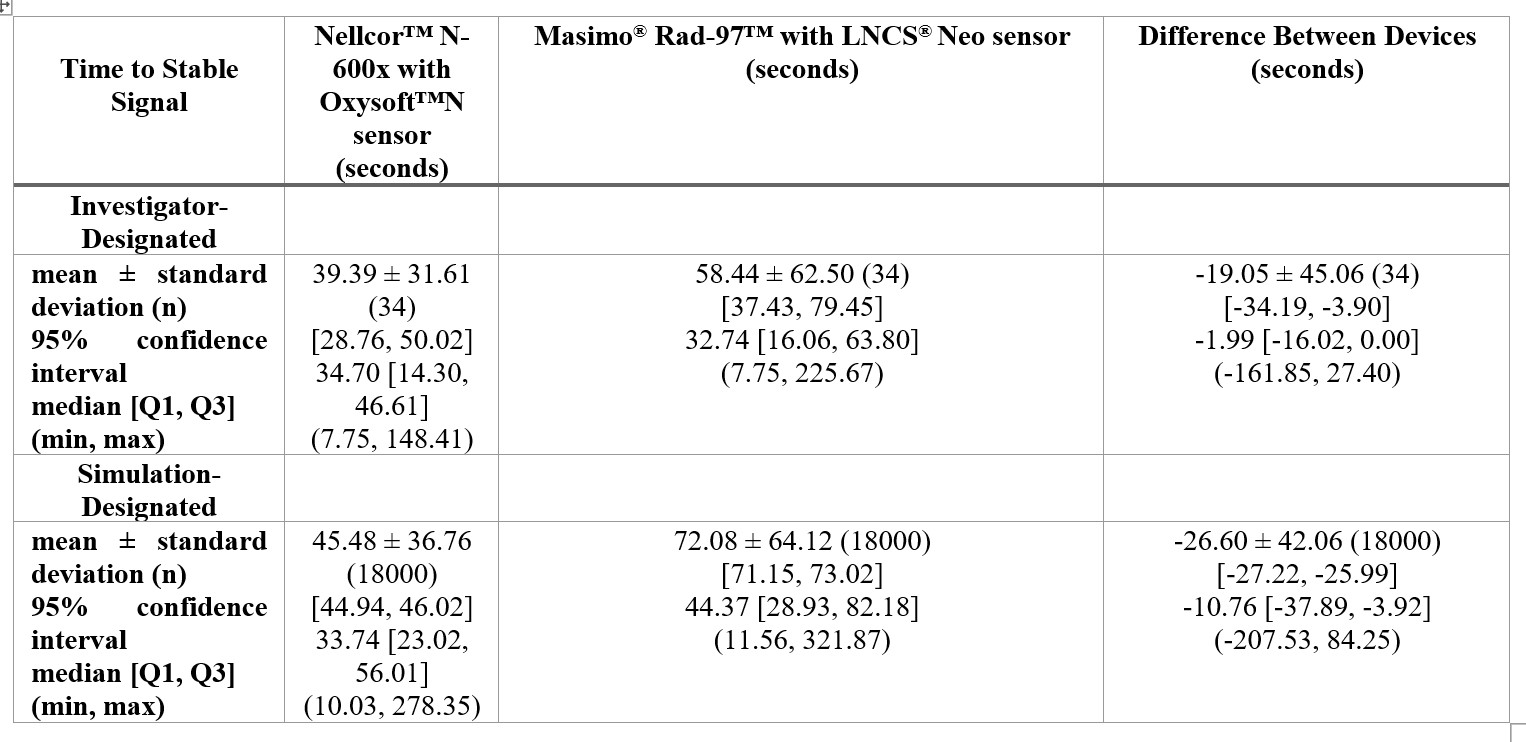
Results: Thirty-four newborns were included in the final analysis (C-section: 30/34). No newborns needed resuscitation. Table 1 summarizes results for time to stable signal for both investigator- and simulation-designated. For both comparisons, Nellcor™ N-600x was found to obtain a stable signal in a significantly shorter time compared to Masimo® Rad-97™. Bland-Altman plots of PR and SpO2 are depicted in Figure 1 and Figure 2, respectively. The observed 95% limits of agreement for Nellcor™ N-600x were smaller than Masimo® Rad-97™, when compared to EKG heart rate. This suggests that, of the two pulse oximeters, Nellcor™ N-600x measured PR had better agreement with EKG heart rate. There appeared to be good agreement in SpO2 readings between the two pulse oximeters for saturations greater than 80.
Conclusion(s): Quicker time to stable signal, and better agreement with EKG heart rate, was reported for Nellcor™ N-600x with OxySoft™ sensor compared to Masimo® Rad-97™ with LNCS® Neo sensor, in newborn infants in the delivery room. Larger studies are needed to confirm these results in preterm infants and in neonates needing resuscitation at birth.
Time to Stable Signal by Device and Measurement Type
Bland Altman Plots of heart rate differences with 2 devices
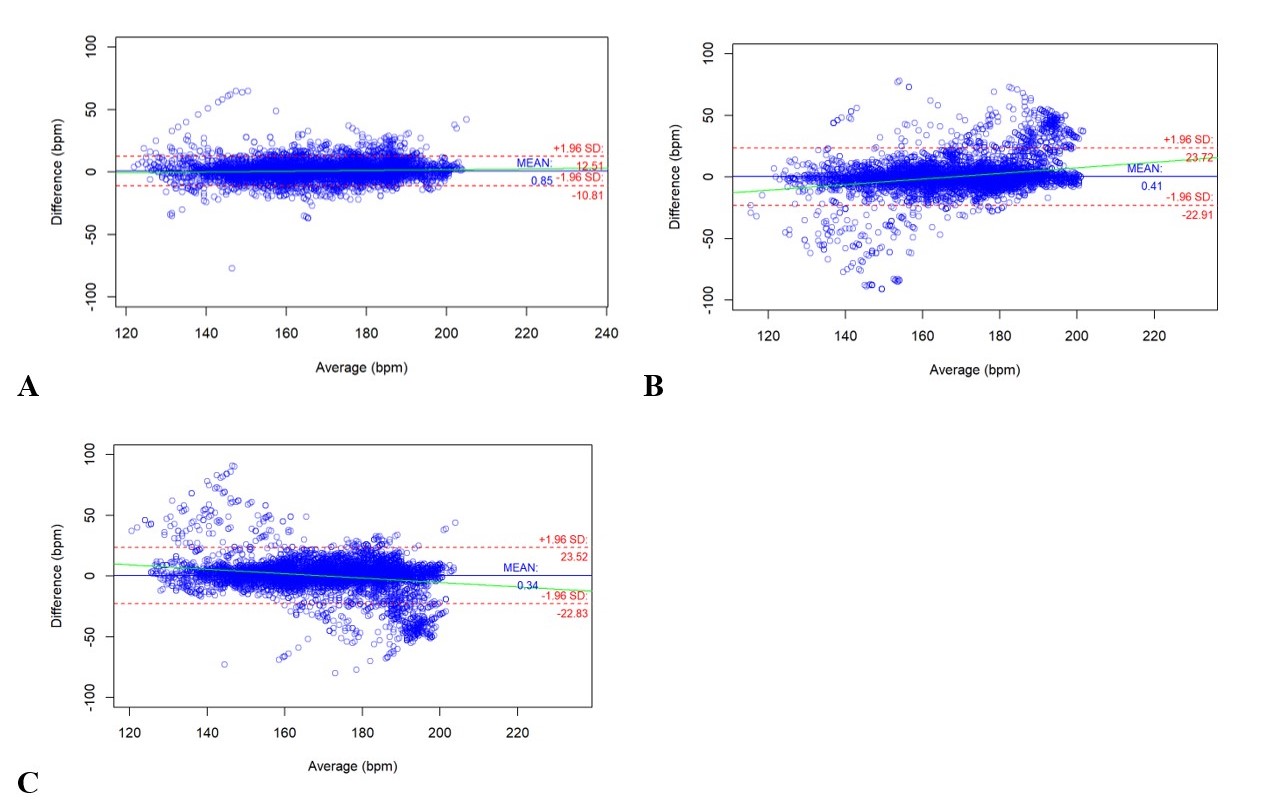 x-axis represents heart rate and the y-axis represents difference in reading between devices.
x-axis represents heart rate and the y-axis represents difference in reading between devices.A. Heart rate reading from EKG compared to Nellcor™ N-600x measured pulse rate
B. Heart rate reading form EKG compared to Masimo® Rad-97TM measured pulse rate
C. Measured pulse rate on Nellcor™ N-600x compared to Masimo® Rad-97™
Bland Altman Plot of SpO2 readings for Nellcor™ N-600x with OxysoftN™ probe and Masimo® Rad-97™ with LNCS®-Neo probe
.jpg) The x-axis represents SpO2 (%) and the y-axis represents difference in reading between devices.
The x-axis represents SpO2 (%) and the y-axis represents difference in reading between devices. 
