Immunizations/Delivery 1
Session: Immunizations/Delivery 1
673 - Nirsevimab uptake among eligible infants in a pediatric primary care network during the 2023-2024 RSV season
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 673.5835
Sarah Schaffer DeRoo, Children's National Hospital, Bethesda, MD, United States; Tamima Hossain, Children’s National Hospital, Manassas, VA, United States; Jessica Lazerov, Children's National Health System, Fairfax, VA, United States

Sarah Schaffer DeRoo, MD, MA (she/her/hers)
Assistant Professor of Pediatrics
Children's National Hospital
Bethesda, Maryland, United States
Presenting Author(s)
Background: Nirsevimab is the first long-acting monoclonal antibody for the prevention of respiratory syncytial virus (RSV) infections to be universally recommended for infants < 8 months and for certain high-risk toddlers. An estimated 44.6% of eligible infants in the United States received nirsevimab during its inaugural year. However, little is known about the characteristics of those infants who received nirsevimab.
Objective: We aimed to determine the nirsevimab uptake rate among eligible infants < 8 months who received care in a network of five pediatric primary care clinics in Washington, DC and to identify the sociodemographic characteristics associated with uptake.
Design/Methods: We determined the nirsevimab uptake rate among infants < 8 months who received care in the clinic network between October 18, 2023 and April 16, 2024 using electronic medical record data. Sociodemographic characteristics were summarized using descriptive statistics. Chi-square tests were used to determine associations between nirsevimab receipt and sociodemographic characteristics.
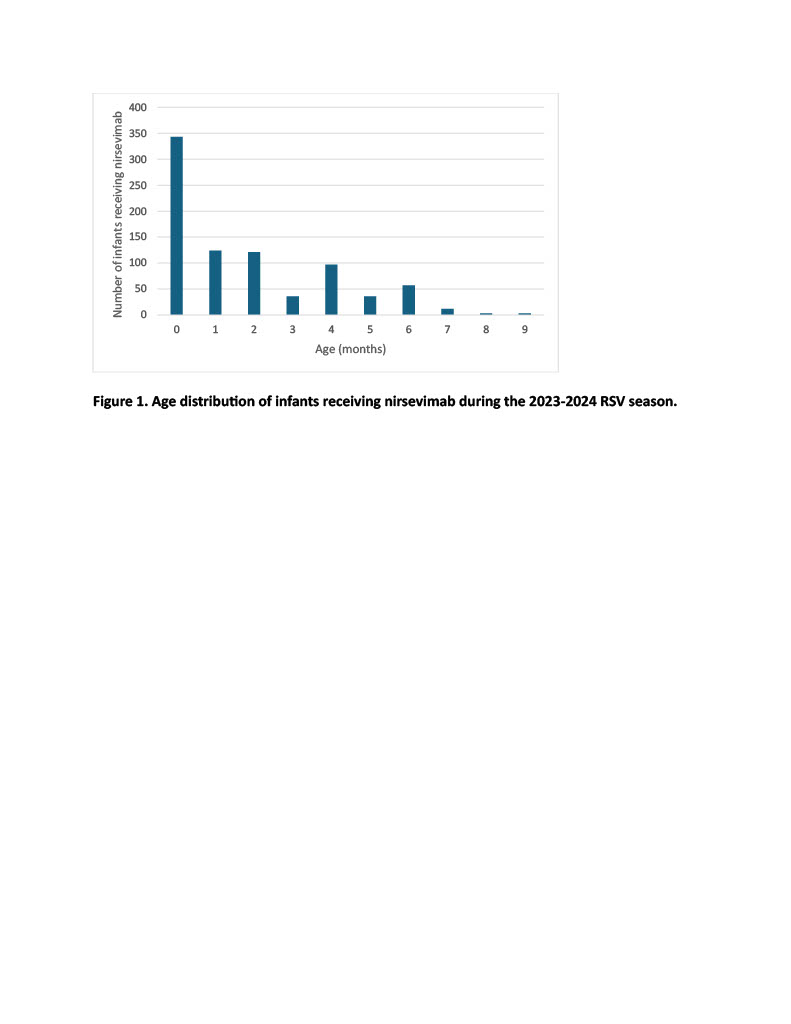
Results: Thirty-eight percent of eligible infants received nirsevimab during the 2023-2024 RSV season. The median age at time of receipt was 1 month. Among infants who received nirsevimab, 69% identified as Black/African American, 15% as White, and 14% as Hispanic/Latino. Sixty-one percent of infants who received nirsevimab had public insurance, while 24% had commercial insurance. Six (0.7%) RSV-immunized infants were diagnosed with bronchiolitis after administration of nirsevimab during the study period, compared with 25 (1.9%) unimmunized infants. Chi-square tests demonstrated significant associations between race (p=0.0008) and insurance type (p=0.0001) with nirsevimab receipt, but not with ethnicity (p=0.24). Bronchiolitis infection due to RSV was negatively associated with nirsevimab receipt (p=0.008).
Conclusion(s): Similar to national estimates, nirsevimab uptake among eligible infants was lackluster, with only 38% uptake. Disparities were identified by race and insurance type; nearly half of eligible White and commercially-insured infants received nirsevimab compared to only one-third of eligible Black/African American and publicly-insured infants. These disparities demonstrate the need for ongoing efforts to understand parents’ perspectives on nirsevimab, factors influencing immunization decision-making, as well as barriers that hinder equitable access to nirsevimab. Further research should elucidate the impact of limited supply on uptake. Our ongoing research efforts aim to compare this data with that from the 2024-2025 RSV season once completed.
Sociodemographic Characteristics of Infants Eligible for Nirsevimab during the 2023-2024 RSV Season
.jpg)
Age distribution of infants receiving nirsevimab during the 2023-2024 RSV season