Infectious Diseases 2: Bacterial infections
Session: Infectious Diseases 2: Bacterial infections
634 - Recurrence of antibiotic resistant infections in children with cancer
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 634.6501
Shane J. Cross, St. Jude Children's Research Hospital, Memphis, TN, United States; Octavio Ramilo, St. Jude Children's Research Hospital, Memphis, TN, United States; Ted H. Morton, St. Jude Children's Research Hospital, Memphis, TN, United States; Joshua Wolf, St. Jude Children's Research Hospital, Memphis, TN, United States

Joshua Wolf, MBBS, PhD (he/him/his)
Member
St. Jude Children's Research Hospital
Memphis, Tennessee, United States
Presenting Author(s)
Background: Children receiving chemotherapy for cancer are at risk of life-threatening antibiotic-resistant bacterial infections. Because febrile neutropenia (FN) following recent antibiotic-resistant infection may be a unique risk period, international guidelines recommend considering initial antibiotic regimens that account for prior resistant infections. However, broader spectrum empiric therapy has significant risks, and not all subsequent episodes are associated with antibiotic resistant infection; some have susceptible infections or no bacterial infection. Therefore, there is uncertainty about appropriate empiric antibiotic regimens during subsequent FN episodes in patients with past resistant infection, and no high quality published data are available for the absolute risk of recurrent resistant bacteria during FN in this patient population.
Objective: We aimed to fill this knowledge gap by evaluating the risk of cefepime-resistant bacterial infections during FN episodes occurring within 6 months after cefepime-resistant bacteremia in pediatric patients with cancer.
Design/Methods: This was an IRB-approved retrospective cohort study of children and adolescents ( < 22 years) with cefepime-resistant Gram-negative bacteremia at St. Jude Children’s Research Hospital identified between 2010 and 2022, comprising data for index bacteremia episodes, and for all subsequent discrete FN episodes within 6 months. We estimated the proportion of FN episodes with cefepime-resistant Gram-negative microbiologically-documented infection and identified risk factors for recurrent resistant infection.
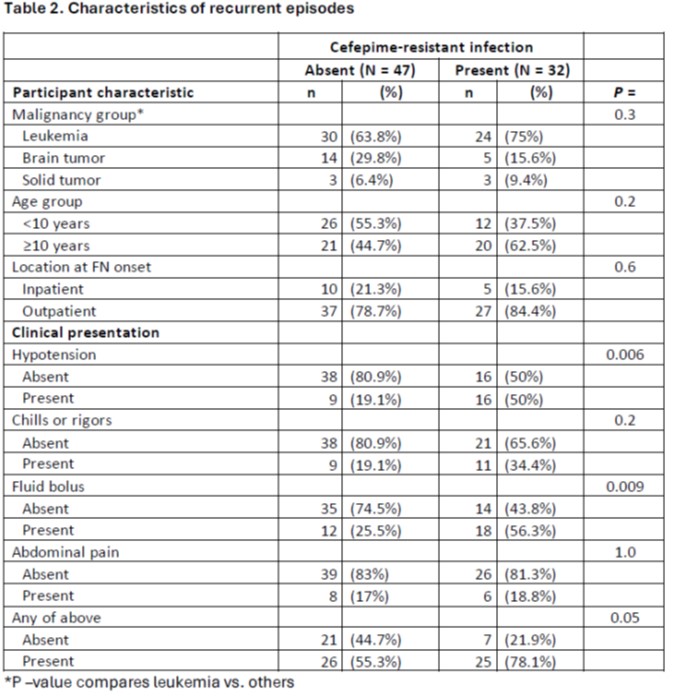
Results: There were 163 episodes in 82 participants; 84 index and 79 recurrent. Descriptive data for demographics, clinical presentation and initial outcome data are shown in Table 1.
Recurrent cefepime-resistant infection occurred in 32/79 (41%) of FN episodes (95%CI 29-51%). Hypotension or fluid bolus were associated with increased risk of cefepime-resistant infection (P = 0.006 and P =0.009 respectively). The absence of signs concerning for severe sepsis (hypotension, fluid bolus, chills/rigors) markedly reduced the risk (10/38 [26%] vs. 22/41 [54%]; P = 0.021).
Conclusion(s): Children and adolescents with cancer presenting with FN after recent cefepime-resistant bacteremia are at high risk of recurrent resistant infection. High-risk features markedly increases risk. Further research to validate these findings, with the aim of developing risk-stratification models could help guide empiric treatment.
Characteristics of included participants
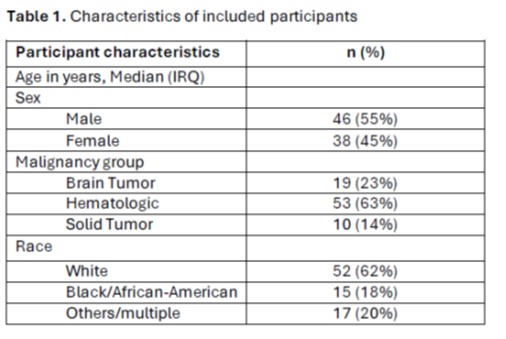
Characteristics of recurrent episodes