Neonatal/Infant Resuscitation 1
Session: Neonatal/Infant Resuscitation 1
293 - Laryngeal Mask Airway as the First Line for Positive Pressure Ventilation in a High Resource Delivery Room Setting
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 293.5706
Kirsten A. Gilliland, Children's Hospital Colorado, Denver, CO, United States; Laura J. Podewils, Denver Health, Denver, CO, United States; Cheri Sims, Denver Health Hospital & Authority, Denver, CO, United States; Karen L. Albrecht, Denver Health, Golden, CO, United States; Danielle Roberts, University of Colorado School of Medicine, Denver, CO, United States

Kirsten A. Gilliland, MD (she/her/hers)
Resident
Children's Hospital Colorado
Denver, Colorado, United States
Presenting Author(s)
Background: In the delivery room (DR), newborn respiratory failure precedes cardiac failure. Ventilation is key and is achieved with multiple interventions. Air leak and suboptimal lung pressure are limitations of face masks (FM) for positive pressure ventilation (PPV). Intubation requires skilled providers for proper insertion. Laryngeal mask airways (LMA) are less invasive and require less training. The 2023 updated NRP guidelines permit LMA use as the primary method for PPV in newborns of gestational age (GA) greater than 34 weeks. Data comparing LMA and FM use for DR PPV is limited.
Objective: Our study describes the implementation of LMA as the first line for DR PPV and determines feasibility, acceptability, and initial outcomes of this practice as standard of care in a high resource, level III NICU with a diverse patient population.
Design/Methods: In August 2023, LMA was introduced as the first line for DR PPV in infants equal to or greater than 35 weeks GA and 1.5 kg. Providers were trained in indications for and insertion of LMA, then assessed on knowledge. They provided written feedback for 1 year following intervention. A 2-year period of all deliveries indicating PPV was analyzed (1). Patient sociodemographic and clinical characteristics as well as neonatal outcomes were compared before and after implementation. Provider pre-post education surveys were collected. Descriptive analyses were used to summarize patient characteristics. Chi-square and ANOVA were used to compare factors across 3 categories: FM pre-intervention, and either FM or LMA as first line for PPV.
Results: There were similar numbers of deliveries requiring PPV pre and post intervention (214 and 213, respectively) (1). Sociodemographic maternal characteristics were also similar (2). During the intervention period, LMA was used as first-line in 58.7% of deliveries. There was a higher percentage of LMA use only or as the first line in infants with lower 1-minute APGAR (p < 0.001) (3). Post-education provider surveys indicated increased knowledge and comfortability regarding LMA use and likelihood to follow the intervention. Feedback also demonstrated easy device accessibility, comfort with insertion, and effective ventilation within 30 seconds of LMA. NICU admission and need for intubation were similar among groups.
Conclusion(s): Our findings indicate that providers of different educational levels successfully inserted LMA after brief training. First line LMA achieved similar outcomes as FM at an urban safety-net hospital. Other institutions may consider adopting LMA as a first line for PPV in the DR.
Image 1: Eligibility Flow Diagram
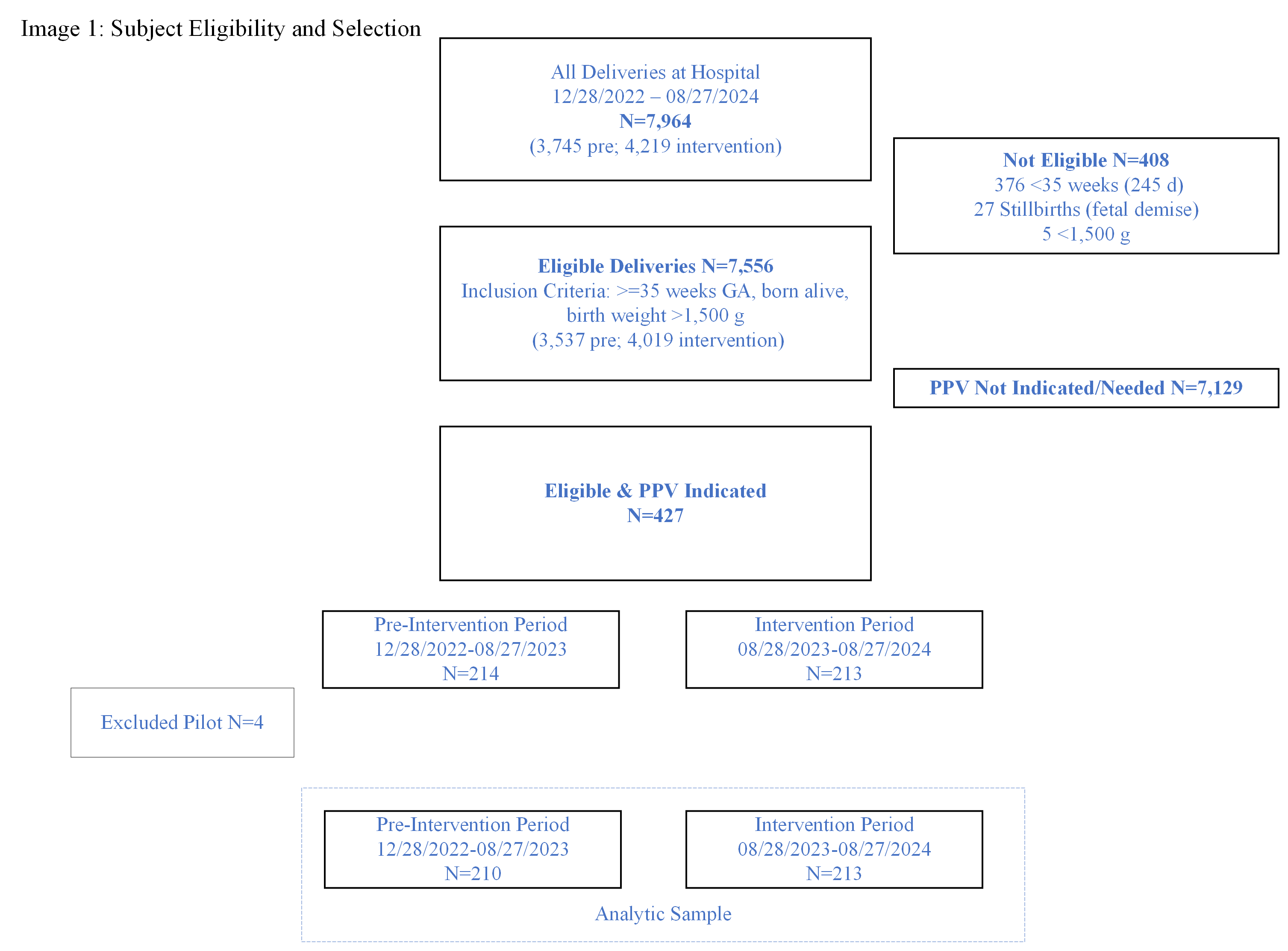 This image depicts the selection criteria for the study and final sample for the pre-intervention and intervention periods.
This image depicts the selection criteria for the study and final sample for the pre-intervention and intervention periods. Image 2. Table 1. Sociodemographic and clinical characteristics of eligible deliveries.
 In comparing pre-intervention eligible and intervention eligible groups, there are no significant differences.
In comparing pre-intervention eligible and intervention eligible groups, there are no significant differences.Image 3: Table 2. Association of a) Intervention Period and b) Use of LMA and Clinical Outcomes.
.png) There is a statistically significant (p < 0.001) difference in providers using LMA only or as first-line with 1 minute APGAR scored 0-3 in the intervention period. Otherwise, there are no statistically significant differences with FM use compared to LMA use for PPV in the analyzed outcomes (5 and 10 minute APGAR scores, NICU admission, intubation, chest compressions, epinephrine use, and HIE).
There is a statistically significant (p < 0.001) difference in providers using LMA only or as first-line with 1 minute APGAR scored 0-3 in the intervention period. Otherwise, there are no statistically significant differences with FM use compared to LMA use for PPV in the analyzed outcomes (5 and 10 minute APGAR scores, NICU admission, intubation, chest compressions, epinephrine use, and HIE). 
