Infectious Diseases 2: Bacterial infections
Session: Infectious Diseases 2: Bacterial infections
623 - Cefepime versus carbapenems for AmpC producing Enterobacterales bacteremia in children
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 623.4207
Takashi Shoji, Tokyo Metropolitan Children's Medical Center, Tachikawa, Tokyo, Japan; Yuto Otsubo, Tokyo Metropolitan Children's Medical Center, Fuchu, Tokyo, Japan; Yuho Horikoshi, Tokyo metropolitan children's medical center, Fuchu, Tokyo, Japan
.jpg)
Takashi Shoji, MD (he/him/his)
Resident
Tokyo Metropolitan Children's Medical Center
Tachikawa, Tokyo, Japan
Presenting Author(s)
Background: Carbapenems are stable against AmpC beta-lactamase, yet cefepime is also recommended for treating AmpC-producing Enterobacterales (AmpC-E) in adults. The efficacy of cefepime for AmpC-E bloodstream infections in pediatric populations, however, remains insufficiently studied.
Objective: To evaluate and compare clinical outcomes associated with cefepime versus carbapenem as definitive therapy for pediatric bloodstream infections caused by AmpC-E.
Design/Methods: This retrospective cohort study was conducted at a tertiary children’s hospital in Japan from May 2010 to August 2024. Eligible patients were < 21 years old with positive blood cultures for AmpC-E, including Enterobacter cloacae complex, Klebsiella aerogenes, Citrobacter freundii, Serratia marcescens, Morganella morganii, and Providencia spp. Definitive therapy was defined as the antibiotic selected after susceptibility determination of the organism. The primary outcome was 30-day mortality, while the secondary outcomes included 30-day recurrence, time to negative blood culture, and treatment toxicity.
Results: Among 65 AmpC-E bacteremia cases, 52 children were included in the analysis. Cefepime and meropenem were used as definitive therapies in 39 (75%) and 13 (25%) cases, respectively. Median age was 11 months (IQR: 3–62), with 53.8% male patients. The isolated organisms included Enterobacter cloacae complex (46.2%), Klebsiella aerogenes (19.2%), Citrobacter freundii (5.8%), Serratia marcescens (28.8%), and Morganella morganii (1.9%). The cefepime group had a higher prevalence of Klebsiella aerogenes (25.6%, p=0.05) and Serratia marcescens (38.5%, p=0.011). The 30-day mortality rate was 5.1% in the cefepime group and 7.7% in the meropenem group (p > 0.99). There were no cases of recurrent bacteremia within 30 days of treatment. Median days to negative blood culture were 2 days (IQR: 1.0–3.0) in the cefepime group and 1.5 days (IQR: 1.0–4.75) in the meropenem group (p=0.757). No cases of treatment-related toxicity, including allergic reactions or Clostridioides difficile infections, were observed in either group.
Conclusion(s): Cefepime and meropenem demonstrated similar clinical and microbiological outcomes for AmpC-E bacteremia in children, suggesting cefepime as a viable carbapenem-sparing option in this setting.
Table1
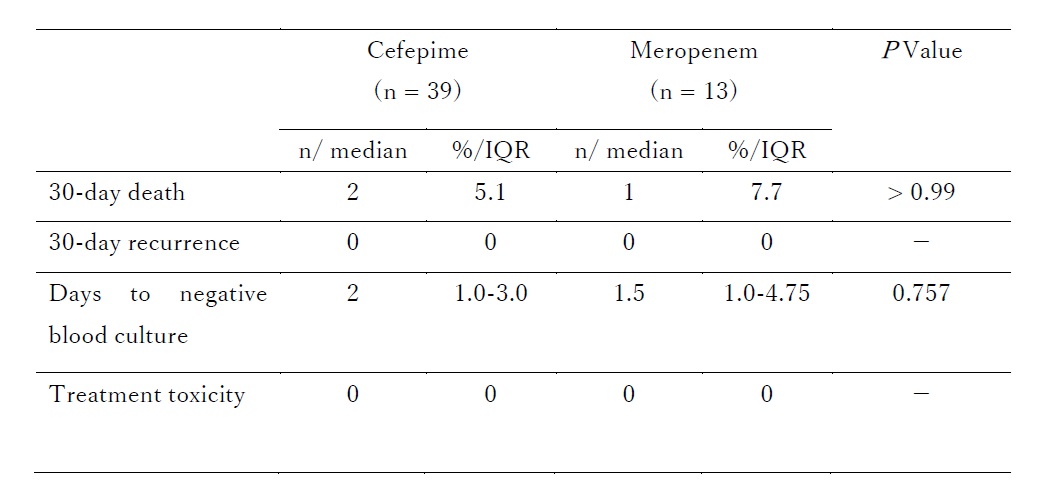 Outcomes
Outcomes
