Neonatal Neurology 3
Session: Neonatal Neurology 3
361 - BOston Neonatal Brain Injury Data for Hypoxic Ischemic Encephalopathy (BONBID-HIE): Lesion Annotation and Neurocognitive Outcome
Friday, April 25, 2025
5:30pm - 7:45pm HST
Publication Number: 361.3948
rina bao, Boston Children's Hospital, Boston, MA, United States; Anna N.. Foster, Boston Children's Hospital, Boston, MA, United States; Rutvi Vyas, Boston Children's Hospital, Boston, MA, United States; Patricia Ellen. Grant, Boston Children's Hospital, Boston, MA, United States; Yangming Ou, Boston Children's Hospital, Boston, MA, United States

Rina Bao, PhD (she/her/hers)
Postdoctoral Research Fellow
Boston Children's Hospital
Boston, Massachusetts, United States
Presenting Author(s)
Background: Hypoxic Ischemic Encephalopathy (HIE) is a clinical syndrome due to a lack of blood flow and oxygen to the brain. It affects around 0.5% of newborns
globally. Despite advancements in Therapeutic Hypothermia (TH), the prognosis for many infants remains challenging, with 35%–50% suffering adverse neurocognitive outcomes by 2 years of age by 2 years of age. Therefore, 63 of the 130 ongoing HIE-related trials worldwide are testing whether new therapies can supplement TH and further reduce adverse outcomes. However, therapeutic innovation is slow and inconclusive, for 1) before therapy, patients at high risk of developing adverse outcomes cannot be identified; 2) after therapy, outcomes cannot be measured until age 2 years. Both issues point to a lack of a neonatal biomarker that can predict adverse 2-year outcomes.
Objective: To facilitate the development of biomarkers in HIE study, we present BOston Neonatal Brain Injury Dataset for HIE (BONBID-HIE), an open-source, comprehensive MRI and clinical dataset from Massachusetts General Hospital (MGH) and Boston Children’s Hospital (BCH). This release contains raw and derived diffusion parameter maps, lesion annotations, as well as NICU outcome and 2-year outcome with 237 patients.
Design/Methods: Data was retrospectively collected from MGH and BCH. Clinical characteristics and demographic information were retrospectively gathered from the electronic health records (EHRs). The clinical variables included maternal information during pregnancy and delivery, as well as infant information. MRIs were acquired on either a GE 1.5T Signa scanner , or, a Siemens 3T TrioTim or PrismaFit scanner.
Results: The data is organized in the format shown in Figure 1. BONBID-HIE provides, per patient: (i) 1ADC ss: skull stripped ADC map; (ii) 2ZADC: ZADC map; (iii) 3LABEL: expert lesion annotations; and (iv) clinical data: clinical variables as written in Table 1A. (v) NICU outcome: status when discharged at NICU, shown in Table 1B. (vi): 2-year outcome: 2-year neurocognitive outcome, noted that patients deceased before 2-year appointment are marked as adverse outcome, as shown in Table 1C. (v) BSID-III scores: Bayle version III scores. Figure 2 shows the ADC map, ZADC map, and expert annotations of example patients with different HIE lesion percentages.
Conclusion(s): This work introduces the BONBID-HIE, an open-source, comprehensive MRI and clinical dataset from Massachusetts General Hospital and Boston Children’s Hospital. It includes data on 237 patients and facilitate developing prognostic biomarkers for further analyses of clinical, treatment, and neurologic outcomes.
Figure 1.
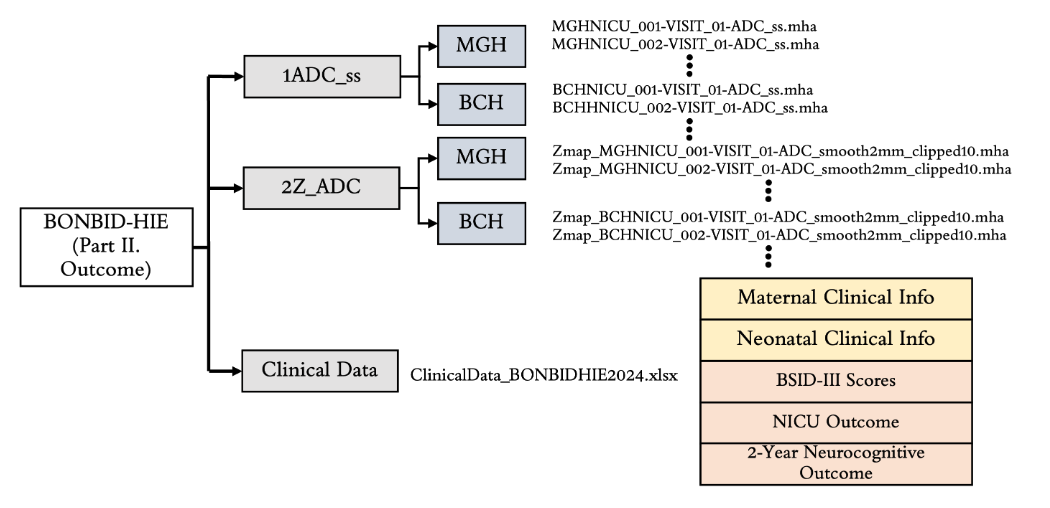 Folder structure of the BONBID-HIE dataset.
Folder structure of the BONBID-HIE dataset.Table 1.
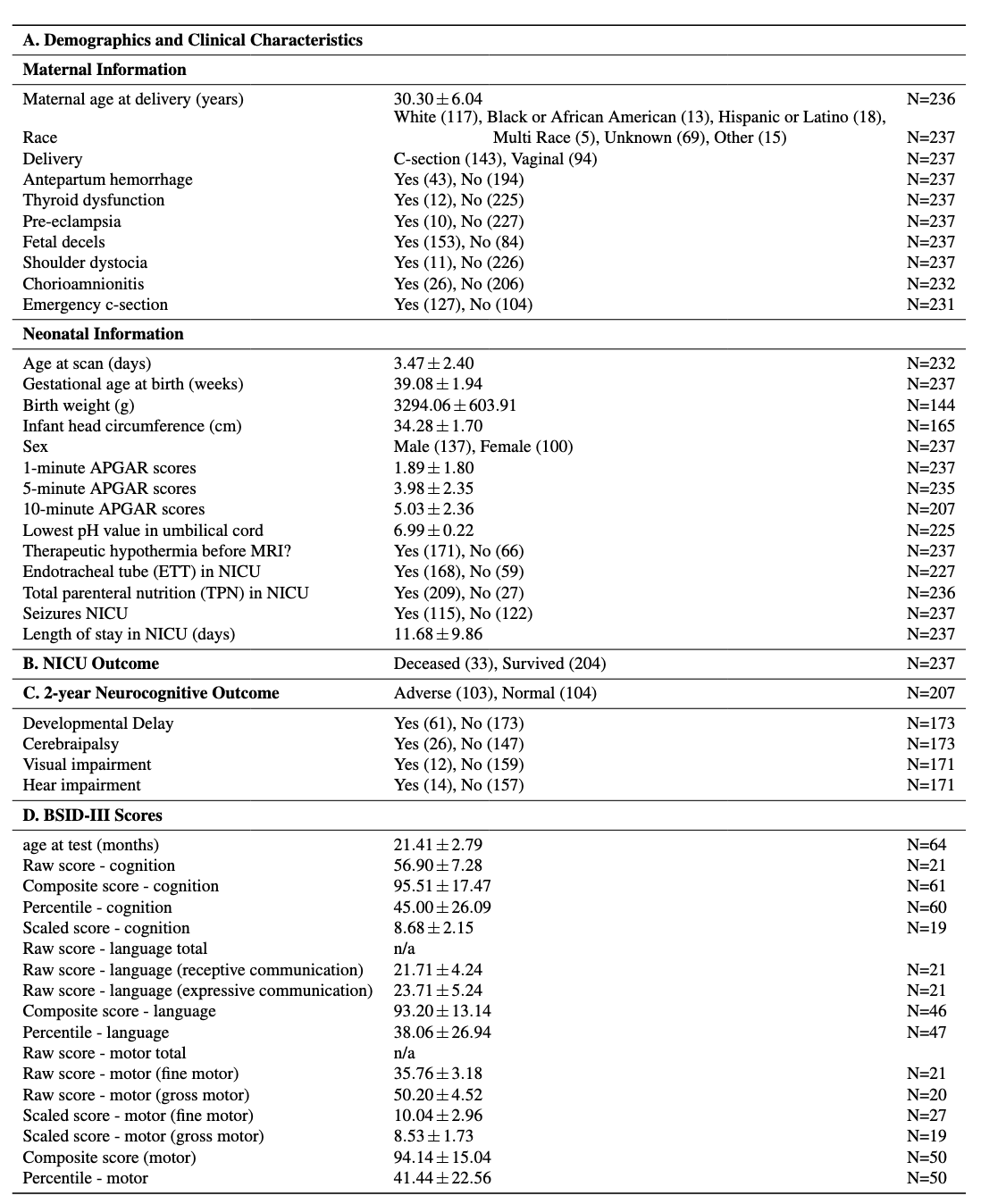 Cohort characteristics (N=237) in MGH and BCH.
Cohort characteristics (N=237) in MGH and BCH.Figure 2.
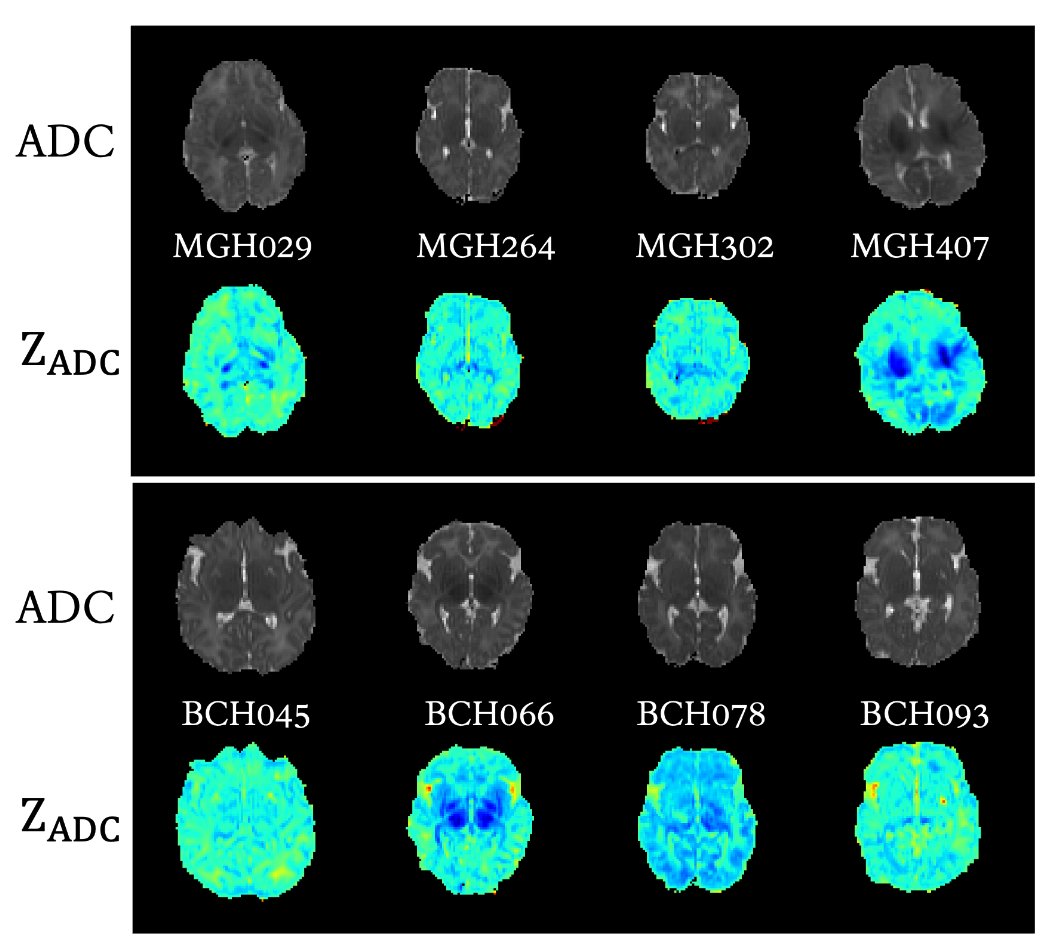 Representative cases for 4 HIE patients from MGH and BCH. For each patient
Representative cases for 4 HIE patients from MGH and BCH. For each patient
