Neonatal Quality Improvement 6
Session: Neonatal Quality Improvement 6
534 - Implementation of the early-onset sepsis calculator in a tertiary care NICU
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 534.5374
Anam Farooqui, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States; Kelli Skvarenina, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States; Shilpa Telang, Mount Sinai Hospital Chicago, Chicago, IL, United States; Elzbieta Kalata, University of Chicago Division of the Biological Sciences The Pritzker School of Medicine, Chicago, IL, United States; Sudhir Sriram, University of Chicago, Chicago, IL, United States; Walid Hussain, Comer Children's Hospital at University of Chicago Medical Center, Chicago, IL, United States

Anam Farooqui, MBBS
Neonatal-Perinatal Medicine Fellow
Comer Children's Hospital at University of Chicago Medical Center
Chicago, Illinois, United States
Presenting Author(s)
Background: Early-onset sepsis (EOS) is a significant cause of neonatal morbidity and mortality. However, out of fear of missing cases, many low-risk newborns may be unnecessarily exposed to empiric antibiotics which can have adverse sequelae. Since its advent, many institutions have adopted the neonatal sepsis calculator to reduce their antibiotic utilization and laboratory testing for EOS.
Objective: Implement this tool in our neonatal intensive care unit (NICU) to decrease EOS evaluations, promote antibiotic stewardship, facilitate maternal-infant bonding, and lower hospital and patient costs.
Design/Methods: A single-center cohort study of all late-preterm and term infants admitted to the NICU, including those exposed to maternal chorioamnionitis (CAM), was conducted using two groups: group 1 from 6/2021-11/2022 (pre-EOS calculator timeframe) versus group 2 from 2/2023-2/2024 (post-EOS calculator timeframe), with a washout period from 12/2022-1/2023. Primary outcome was proportion of infants receiving antibiotics. Secondary outcomes were NICU and hospital LOS, duration of antibiotics, number of CAM infants receiving antibiotics, and rates of blood culture draws and treatment for culture-negative sepsis. Bi- and multivariate analyses were performed with statistical significance defined as p-value < 0.05.
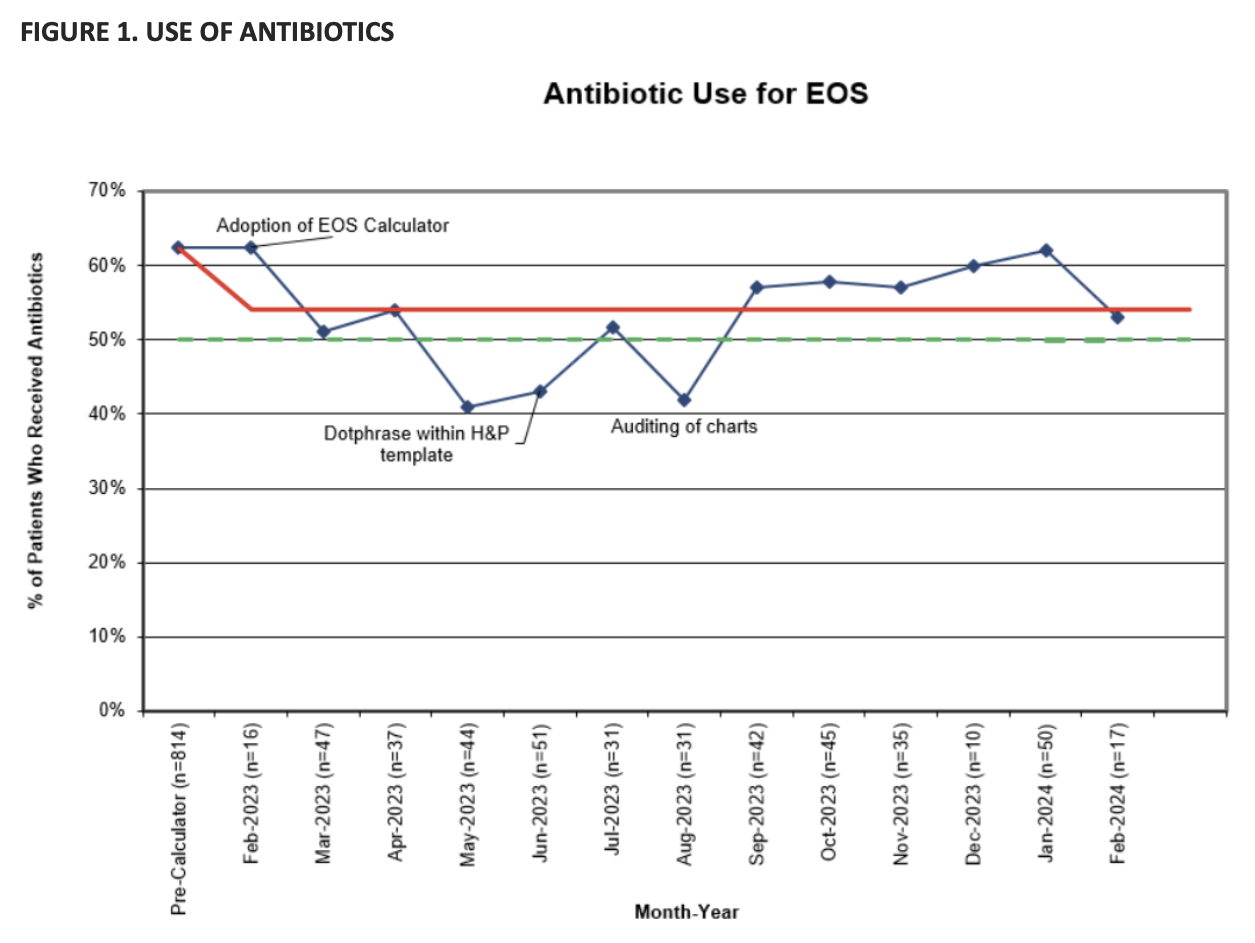
Results: 1306 infants, with similar demographics, were included: 814 in group 1 and 492 in group 2 (Table 1). Following roll-out of the calculator, antibiotic use (Figure 1) decreased from 62% to 51% (p 0.00) with reduction in odds by 40% (95% CI 0.47-0.75) even after controlling for CAM. In the CAM subgroup, antibiotic use decreased from 99% to 50% (p 0.00). There was reduction in days of therapy per 1000 NICU (168 vs. 110, p 0.00) and total (93 vs. 57, p 0.00) patient days. Fewer blood cultures were drawn (84 vs. 67%, p 0.00) with a decrease in infants being treated for culture-negative sepsis (7 vs. 3%, p 0.00). NICU length of stay (LOS) decreased by 1 day (p 0.00) with a reduction in odds by 15% (95% CI 0.77-0.94) even after controlling for CAM and use of antibiotics. Based on hospital estimates, this would be equivalent to savings of $916,000-1.84 million for 1,000 NICU patients in direct costs, and savings of $5.82-12.5 million for 1,000 NICU patients in charges. Hospital LOS decreased by 10% (95% CI 0.82-0.97) after controlling for gestational age.
Conclusion(s): Our testing, antibiotic use and hospitalization for EOS were effectively decreased, with substantial savings in patient costs and charges. No significant negative effects were found, with similarly low rates of positive cultures in both groups.
Table 1. Characteristics and outcomes
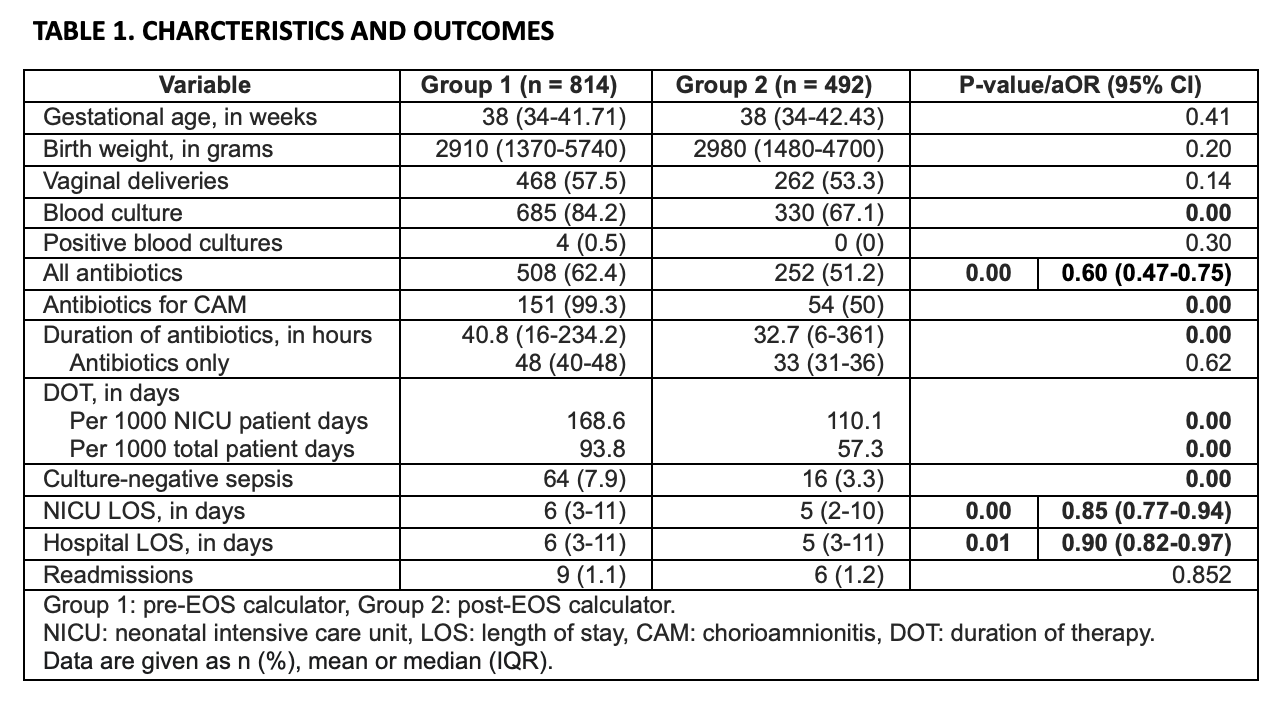
Figure 1. Use of antibiotics