Infectious Diseases 4: Improving antibiotic use
Session: Infectious Diseases 4: Improving antibiotic use
140 - Interventions to Minimize Unnecessary Antibiotic Use for Acute Otitis Media: A Meta-Analysis
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 140.5331
Theresa Morin, Intermountain Health, Denver, CO, United States; Rana E. El Feghaly, Children's, Kansas City, MO, United States; Amanda Nedved, Children's Mercy Hospitals and Clinics, Lenexa, KS, United States; Sophie E. Katz, Vanderbilt University School of Medicine, Nashville, TN, United States; Amy B. Stein, Denver Health, Denver, CO, United States; Timothy Jenkins, Denver Health, Denver, CO, United States; Heather Laferriere, Vanderbilt University, Nashville, TN, United States; Amy Keith, Denver Health and Hospital Authority, Denver, CO, United States; Holly M. Frost, Intermountain Health and University of Utah, Arvada, CO, United States
- TM
Theresa Morin, MA (she/her/hers)
Research Manager
Intermountain Health
Denver, Colorado, United States
Presenting Author(s)
Background: Acute otitis media (AOM) is the leading reason antibiotics are prescribed to children. Despite guidelines advocating for watchful waiting and shorter antibiotic durations, over-prescribing continues to be a national concern. Addressing the cause of this issue is critical to minimize healthcare burden from adverse drug events, antibiotic resistance, and resource depletion in times of antibiotic shortage.
Objective: To quantify the potential reduction in antibiotic days of therapy (DOT) for AOM if prescribers adhered to national guidelines and to identify antibiotic stewardship interventions that effectively reduce antibiotic DOT for AOM.
Design/Methods: We conducted a systematic review and meta-analysis of studies from the United States, Canada, and Europe, published between 2000 and 2023. The review sourced Cochrane databases for studies focusing on diagnostic accuracy, antibiotic duration, and watchful waiting in children aged 6 months to 17 years with uncomplicated AOM. We extracted and analyzed data to estimate the pooled prevalence of AOM outcomes and the effectiveness of various stewardship interventions. Using these estimates, we conducted a simulation study to establish DOT for AOM in current practice and following the American Academy of Pediatrics (AAP) and United Kingdom's National Institute for Health and Care Excellence (NICE) guidelines.
Results: The meta-analysis included 78 studies. In total, an estimated 107 million DOT are prescribed to children in the United States annually for AOM. Our simulation study found that following AAP guidelines could reduce DOT by 60.6 million days (56%). Adherence to NICE guidelines could reduce DOT by 76.7 million (71%). Unnecessary DOT were most commonly due to incorrect diagnosis (53.1 million, 49%), immediate antibiotic use (24.6 million, 23%), and longer than recommended antibiotic durations (6.8 million, 6%). Interventions aimed at increasing watchful waiting and decreasing duration of antibiotics were the most effective in reducing unnecessary DOT, with a 23% and 31% reduction respectively.
Conclusion(s): Adherence to national guidelines for AOM management could significantly reduce unnecessary antibiotic exposure in children, potentially averting millions of antibiotic days annually. This underscores the urgent need for uptake of scalable interventions that have demonstrated success in reducing antibiotic overuse. Broadly implementing interventions that utilize shorter courses of antibiotics and watchful waiting strategies could transform pediatric care, reducing the burden of antibiotic resistance and improving health outcomes for children.
Figure 1.
.png) Total days of therapy based on 11.5 million annual AOM encounters and adherence to guidelines. The confidence intervals are calculated based on the range of AOM encounters (5.2 million and 19.2 million), and the range of days of therapy in AAP recommendations and NICE guidelines.
Total days of therapy based on 11.5 million annual AOM encounters and adherence to guidelines. The confidence intervals are calculated based on the range of AOM encounters (5.2 million and 19.2 million), and the range of days of therapy in AAP recommendations and NICE guidelines.Figure 2.
.png) Odds ratio (OR) plot showing the pooled OR and 95% CI for the effectiveness of three interventions to reduce the use and prescribing of antibiotics. Watchful waiting was considered in 9 studies, duration of therapy in 2 studies, and diagnostic accuracy in 4 studies.
Odds ratio (OR) plot showing the pooled OR and 95% CI for the effectiveness of three interventions to reduce the use and prescribing of antibiotics. Watchful waiting was considered in 9 studies, duration of therapy in 2 studies, and diagnostic accuracy in 4 studies.Table 1.
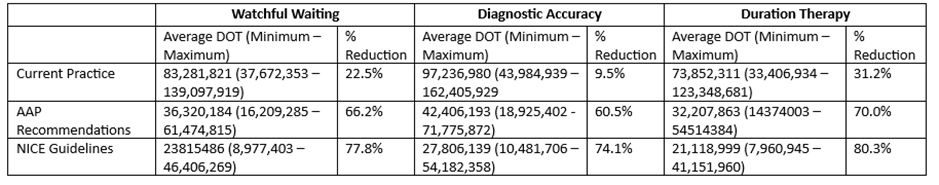 Table of the reduction in days of therapy for three effectiveness interventions on current practice. These calculations assume a base rate of ~6% change in prescribing rate without intervention. The table gives the estimated days of therapy under current practice and the guidelines. The percent reduction represents the change from current practice estimate.
Table of the reduction in days of therapy for three effectiveness interventions on current practice. These calculations assume a base rate of ~6% change in prescribing rate without intervention. The table gives the estimated days of therapy under current practice and the guidelines. The percent reduction represents the change from current practice estimate.
