Neonatal Nephrology/AKI 2
Session: Neonatal Nephrology/AKI 2
026 - Nephrotoxin Exposure as a Modifiable Risk Factor for Neonatal Acute Kidney Injury: Results of a Multicentre Observational Cohort Study in Australia and New Zealand (NeoKANZ)
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 26.5813
Amanda Dyson, The Canberra Hospital, canberra, Australian Capital Territory, Australia; Eveline Staub, Royal North Shore Hospital, St Leonards, New South Wales, Australia; Sunaina Nundeekasen, The Canberra Hospital, canberra, Australian Capital Territory, Australia; Tim Schindler, Royal Hospital for Women, Sydney, New South Wales, Australia; Angelica Allermo Fletcher, Wellington Regional Hospital, Wellington, Wellington, New Zealand; Helen G. Liley, The University of Queensland, South Brisbane, Queensland, Australia; Alison L. Kent, University of Rochester, NY, USA, Henley Beach, South Australia, Australia
- AD
Amanda Dyson, MBBCh FRACP MMed (Clin Epi) CCPU (she/her/hers)
Neonatologist
The Canberra Hospital
canberra, Australian Capital Territory, Australia
Presenting Author(s)
Background: Neonatal acute kidney injury (AKI) is associated with adverse short- and long-term outcomes including an increased risk of mortality, morbidity during NICU admission and increased risk of developing chronic kidney disease. Nephrotoxic medication (NM) exposure is a potentially modifiable risk factor for AKI in this population.
Objective: The aim of this study was to assess the proportion of neonates exposed to NM and its association with AKI in the first 7 days of life (DOL) in an Australian and New Zealand setting
Design/Methods: Retrospective chart review of all neonates admitted < 14 days of age to 5 perinatal and surgical neonatal intensive care units (NICUs) in Australia and New Zealand from 1/1/2019 to 6/30/2019. Exclusion criteria included: death < 48hrs of age, intravenous fluid therapy < 48 hrs, severe congenital kidney or urinary tract abnormalities, lethal chromosomal anomalies, congenital heart disease (surgical repair or prostaglandin infusion in the first 7 DOL.) Data were collected on demographics, morbidity, mortality and kidney function during NICU admission. We defined AKI using modified KDIGO criteria (increase in serum creatinine of at least 27umol/l or 50% above previous lowest value, or urinary output of less than 1ml/kg/hour). NM’s included antibiotics, antivirals and COX inhibitors and exposure was considered if receiving one or more NM.
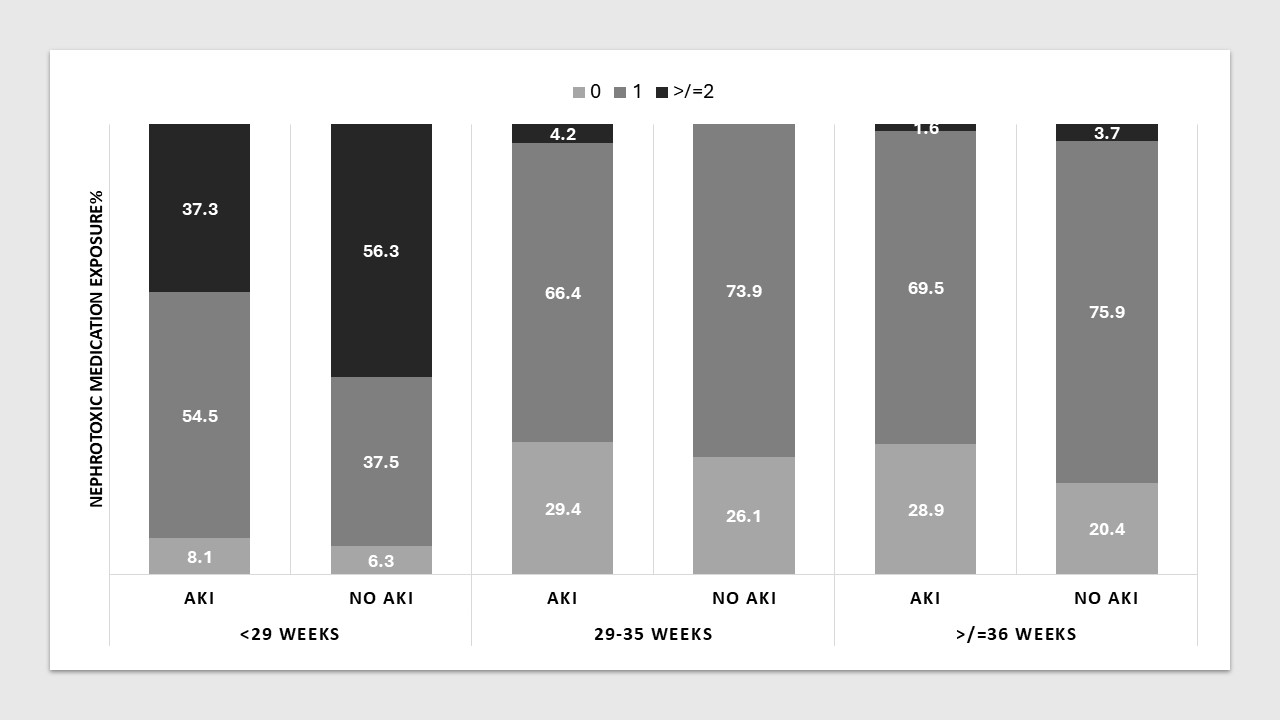
Results: Of 794 included neonates, 581 (73.2%) had sufficient data to determine AKI status in the first 7 DOL. Median gestational age (GA) was 33.3 weeks (range 23.0-42.1), median birth weight 1890g (420-5020g) (table 1). Of these, 116 (20.0%) had AKI (13.9% with GA < 29 weeks, 16.2% with GA 29-35 weeks, 29.7% with GA ≥36 weeks). Of the 581 neonates, 139 (23.9%) had no exposure to NM in the first week, 328 (65.7%) were exposed to one, and 60 (10.3%) to two or more (figure 1). Aminoglycoside antibiotics were the most common NM class administered to the whole cohort (75.4% of infants), followed by COX inhibitors (7.4%) and glycopeptide antibiotics (3.6%). There was no significant association between the exposure to one or more NM and AKI in any of the three GA groups ( < 29 weeks p=0.38, 29-35weeks p=0.38, ≥36 weeks p=0.29).
Conclusion(s): Neonates of all GA requiring NICU care frequently receive NM, especially aminoglycoside antibiotics. Those born < 29 weeks are more commonly exposed to two or more NM. In this cohort, NM exposure does not appear a modifiable risk factor for AKI in the first 7 DOL.
Table 1: Demographic characteristics of newborns with sufficient data to determine AKI status in the first week of life
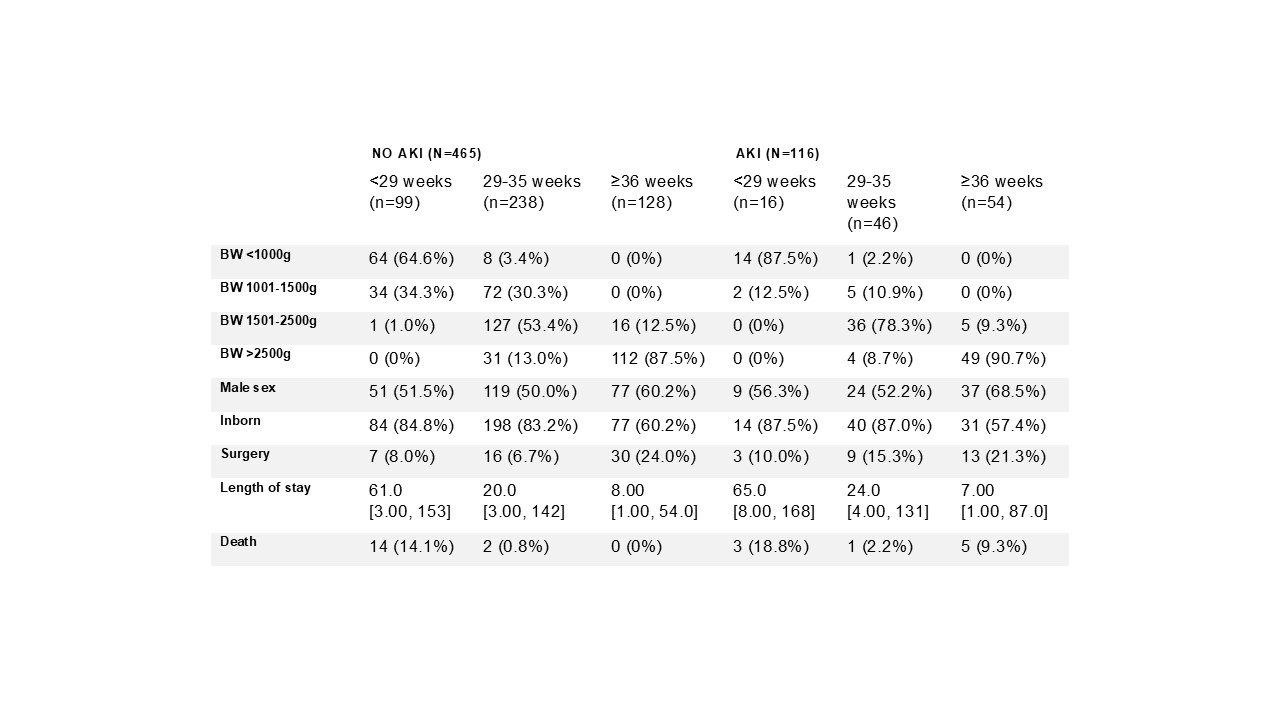 BW: birth weight. Numbers are n with percent in brackets, or median with range in brackets.
BW: birth weight. Numbers are n with percent in brackets, or median with range in brackets. Figure 1: Proportion of newborns exposed to nephrotoxic medication in the first week of life by gestational age group and AKI status