Medical Education 4: Technology and Simulation
Session: Medical Education 4: Technology and Simulation
215 - Development of Realistic 3D Silicone Molds for Ultrasound-Guided IV Placement Training in Neonates
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 215.4470
Shaylin Dalton, University of the Incarnate Word School of Osteopathic Medicine, San Antonio, TX, United States; pratik parikh, Baylor College of Medicine, San Antonio, TX, United States; Aarnav Parikh, Basis Texas Charter School, San Antonio Shavano Campus, San Antonio, TX, United States; Aneesha Morris, Baylor College of Medicine, San Antonio, TX, United States

Shaylin Dalton, BA
MS-II
University of the Incarnate Word School of Osteopathic Medicine
San Antonio, Texas, United States
Presenting Author(s)
Background: Neonates frequently require intravenous (IV) access for life-sustaining treatments. Ultrasound (US)-guided IV placement has become crucial in these cases, improving insertion accuracy, reducing trauma, and increasing success rates for neonates with difficult vascular access. Traditional training methods, including classroom learning or adult phantoms, fail to replicate the anatomical and physiological specifics of neonates, creating a need for neonatal-specific training models. Three-dimensional (3D) silicone molds provide a valuable training tool, with potential to closely simulate neonatal tissue and vein characteristics.
Objective: To develop a 3D silicone mold designed for training in both peripheral and central US-guided IV placement in neonates.
Design/Methods: This study involved the iterative development of three-dimensional (3D) silicone molds, starting with the design and 3D printing of molds using PLA filament as casting forms (Figure 1). These forms were used to create silicone models with embedded synthetic veins. Various ratios of Dragon Skin™ liquid silicone parts (Part A and Part B) were tested. Modifications included the addition of a silicone softener, and talcum powder to improve texture, elasticity, and ultrasound imaging properties. Silicone tubing with internal diameters of 0.2 mm and 0.4 mm were embedded in the molds to simulate neonatal veins. Feedback on mold realism and effectiveness was gathered through a survey of nurse practitioners, nurses, and neonatologists.
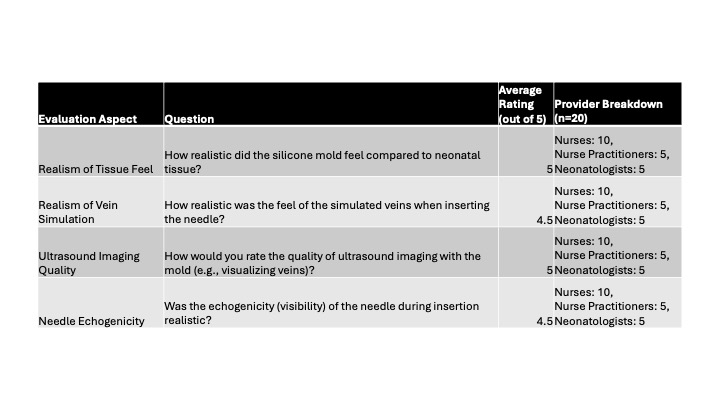
Results: The initial 1:1 silicone compound ratio produced a mold that was too rigid, unlike neonatal tissue. Adjustments to a 1:1.2 ratio with silicone softener achieved a more elastic, tissue-like consistency, although needle echogenicity remained suboptimal. Further refinement to a 1:1.3 silicone compound ratio with 3% talcum powder and silicone softener produced molds with improved tactile realism and echogenicity, enabling clear differentiation between tissue structures and simulated veins on ultrasound imaging (Figure 2). In surveys, almost all providers agreed that the silicone molds exhibited realistic and effective characteristics for training in US-guided IV access (Table 1).
Conclusion(s): The iterative development process led to the creation of highly realistic 3D silicone molds for US-guided IV placement training in neonates. These molds will form the basis of a training module aimed at educating neonatal and pediatric providers in both ultrasound-guided line placement, ultimately enhancing clinical competency and neonatal care outcomes.
Figure 1: 3-Dimensional PLA mold development and final product
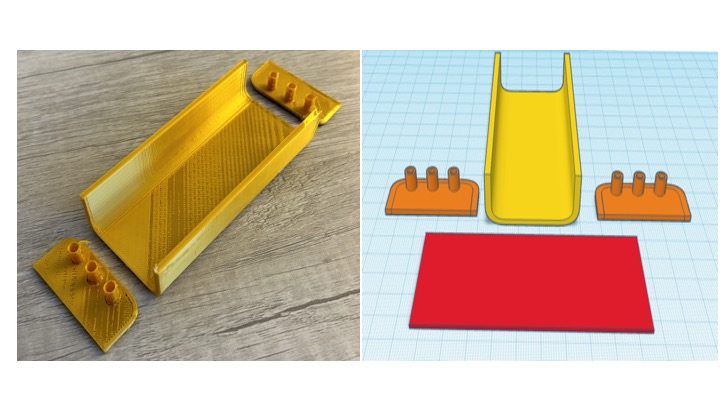 Gold PLA filament with infill of 20% created 10cm x 5cm mold with tunneled sides and a rectangular cover.
Gold PLA filament with infill of 20% created 10cm x 5cm mold with tunneled sides and a rectangular cover.Figure 2: Silicone mold with various consistency.
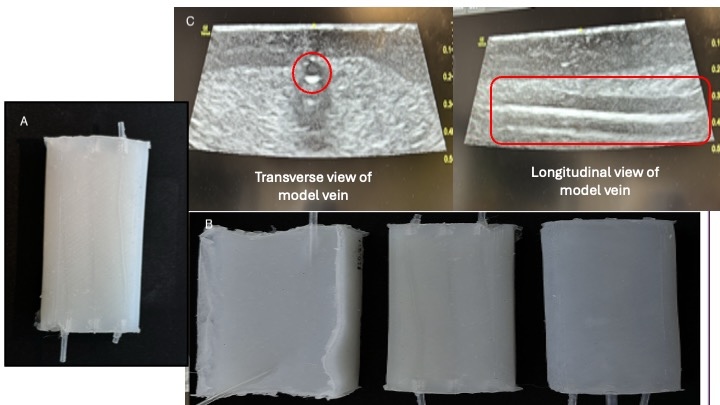 A) Final 1:1.3 silicone mold with 3% talcum powder and silicone softener. B) Various iterations of silicone molds. C) Ultrasound image showing tissue and vein in different views.
A) Final 1:1.3 silicone mold with 3% talcum powder and silicone softener. B) Various iterations of silicone molds. C) Ultrasound image showing tissue and vein in different views. Table 1: Silicone mold provider survey.