Neonatal Quality Improvement 4
Session: Neonatal Quality Improvement 4
510 - Safe and effective use of intranasal sedation (INS) to decrease general anesthesia (GA) for neonatal magnetic resonance imaging (MRI) studies.
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 510.6181
Sarah M. Sanchez, Maria Fareri Children's Hospital at Westchester Medical Center, Fort Lee, NJ, United States; Anita Ware, Maria Fareri Children's Hospital at Westchester Medical Center, Rye Brook, NY, United States; Diana D'Agrosa, Westchester Medical Center, Yonkers, NY, United States; De Borah Briston, WMCHealth, Vahalla, NY, United States; Gabrielle Hodson, Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, United States; Meenakshi Singh, Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, United States
- SS
Sarah M. Sanchez, MD, FAAP (she/her/hers)
Neonatal-Perinatal Medicine Fellow
Maria Fareri Children's Hospital at Westchester Medical Center
Fort Lee, New Jersey, United States
Presenting Author(s)
Background: Pharmacologic sedation in neonates is associated with short & long-term adverse effects. Our NICU had no protocol for MRI with sedation, which led to high failure rate of feed & swaddle (F&S) MRI & high percentage of general anesthesia (GA) for MRI. An IRB exempt Quality Improvement (QI) project led to equitable improvement in successful F&S MRI rate but no consistent reduction in GA. A 2nd phase was launched, using INS for eligible NICU patients with goal of reducing GA for NICU MRI.
Objective: We created the following SMARTIE aims:1.Safe & efficacious use of INS for NICU MRI with ≥80% success & ≤ 10% having respiratory side effects over 6 months across all races, 2.Decrease GA use for NICU MRI by 10% from baseline over 6 months across all races, 3.Decrease NICU MRI wait time by 10% from baseline over 6 months across all races.
Design/Methods: We created a multidivisional team of key stakeholders from NICU, Pharmacy, Radiology & Pediatric Anesthesia. A standardized protocol for F&S MRI showed success rate improvement from baseline 76% to >90% from 2021-end 2023. While improvement was seen in all races, white race saw the most improvement. The rate of GA use & study wait time did not see sustained improvement. This led to 2nd phase; INS for eligible patients & studies. INS for procedural sedation was incorporated into hospital policy. Applicable staff underwent moderate sedation credentialing. A protocol for providing INS for MRI studies was developed & revised based on numerous PDSA cycles. On August 1, 2024 INS for NICU MRI went “live”. A debrief form, completed after each INS study is a process measure. Any respiratory compromise during INS is monitored as a balancing measure. Operational definition for major respiratory event is apnea/brady/desat event requiring vigorous stimulation or PPV.
Results: All INS studies for eligible infants were successful in the first 3 months (6 studies total) (Table1). This included 1 patient with cleft lip & palate, a known risk factor for difficult intubation. 1 patient had desaturation requiring brief support with nasal cannula. None had major respiratory event.All INS cases have been followed by a debrief form (100% process measure). INS for MRI studies has significantly decreased GA use (Fig 1).INS study duration averaged 78min vs ~30min F&S, yet study wait time was 32hrs, lower than average 41hrs historically (Fig 2).
Conclusion(s): INS use led to successful completion of longer & multiple types of MRI studies, led to reduction in GA use & is well tolerated with no major respiratory compromise. INS has potential for expansion into other areas of children’s hospital.
Table 1: NICU MRI Intranasal Sedation (INS) Cases August to October 2024.
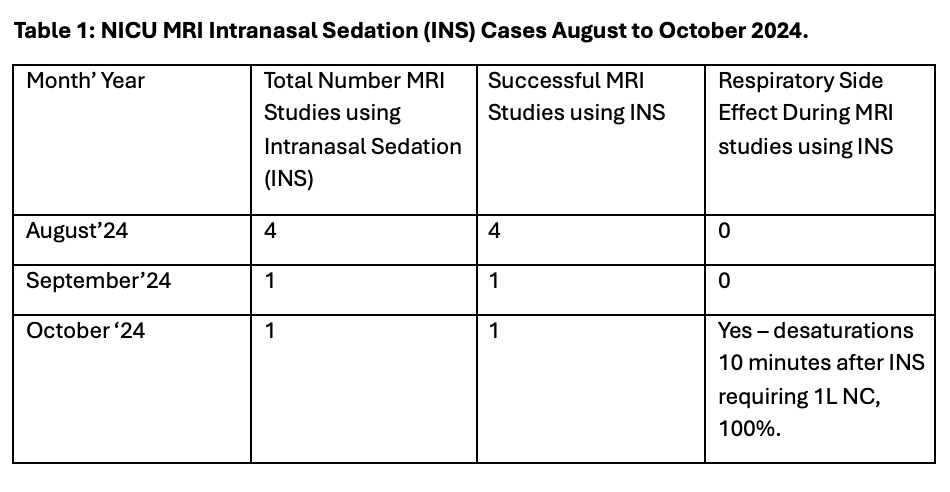
Figure 1: Control (p) Chart for NICU MRI Under General Anesthesia (GA) October 2021 to October 2024.
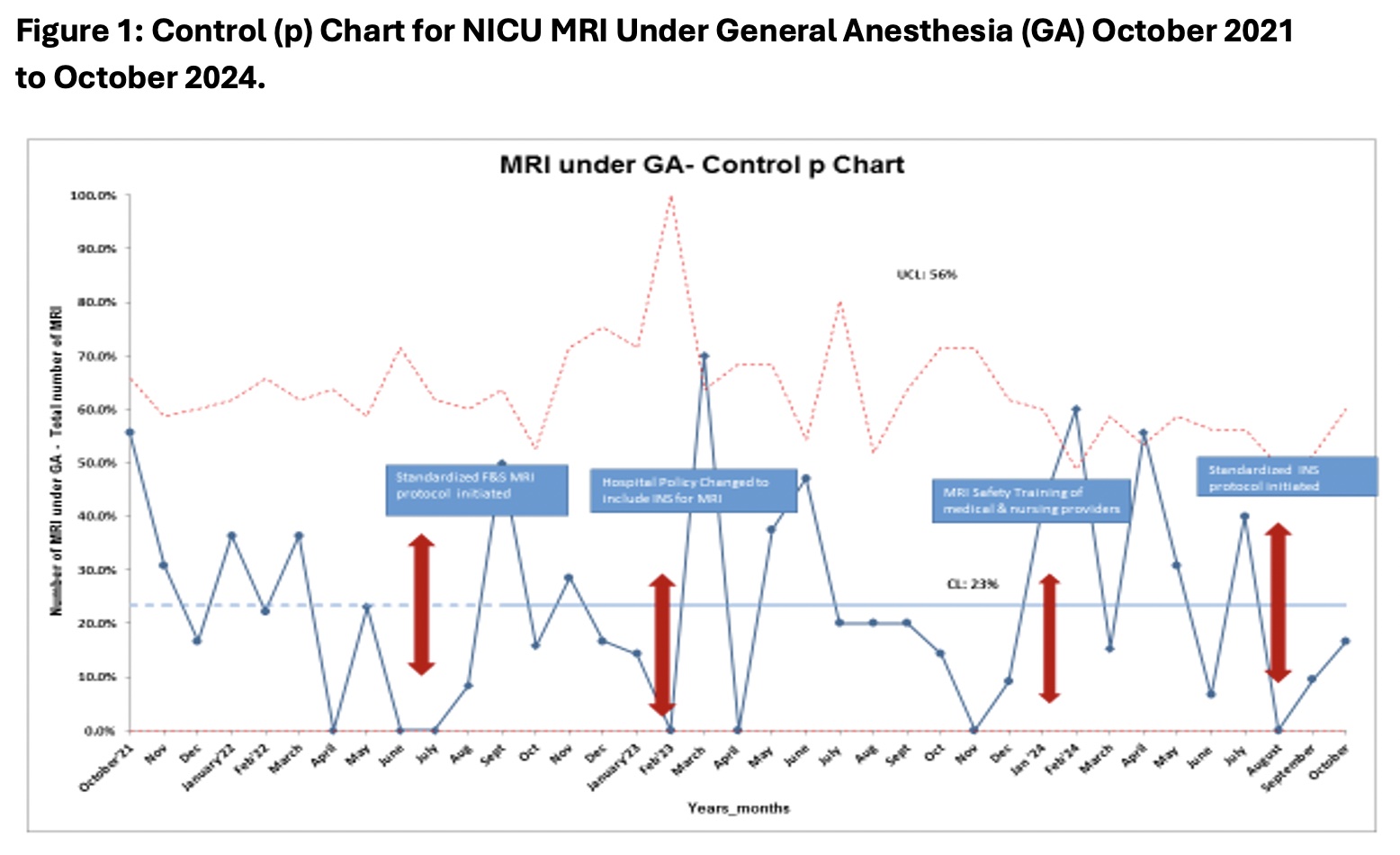
Figure 2: Control Chart (Xbar and S) for MRI study wait time October 2021 to October 2024.
.jpg)


