Quality Improvement/Patient Safety 5
Session: Quality Improvement/Patient Safety 5
480 - Reducing Erroneous Penicillin Allergy Labels (REPeAL) in Pediatric Urgent Care Clinics
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 480.6202
Jennifer McKinsey, Children's Mercy Kansas City, Overland Park, KS, United States; Rana E. El Feghaly, Children's, Kansas City, MO, United States; Sheryl A. Chadwick, Children's Mercy Kansas City, Kansas, MO, United States; Erin Hilton, Children's National Health System, Liberty, MO, United States; Jen Olsen, Children's Mercy Hospitals and Clinics, Overland Park, KS, United States; Katherine Salsbury, Children's Mercy Hospitals and Clinics, Olathe, KS, United States; Tiffany Addington, Children's Mercy Kansas City, LEES SUMMIT, MO, United States; Holly Austin, Children's Mercy Hospitals and Clinics, Leawood, KS, United States; Maria V. Blanco, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Emily Cox, Children's Mercy Hospitals and Clinics, Odessa, MO, United States; Todd Ehrlich, Children's Mercy Hospitals and Clinics, Olathe, KS, United States; Emily J. Montgomery, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Amanda Nedved, Children's Mercy Hospitals and Clinics, Lenexa, KS, United States

Jennifer McKinsey, MD
Clinical Associate Professor of Pediatrics
Children's Mercy Kansas City
Overland Park, Kansas, United States
Presenting Author(s)
Background: 10% of the United States population reports adverse drug reactions (ADRs) to penicillin, yet only 1% has true hypersensitivity. Penicillin allergy labels (PALs) are associated with worse health outcomes.
Objective: To decrease the rate of PALs in our pediatric urgent care (UC) from 7.1% to 5.0% over 12 months.
Design/Methods: A multidisciplinary team of UC clinicians and nurses, a family advisor, quality improvement (QI) and Infectious Disease specialists used QI tools and methodology to develop interventions that included education, a risk stratification algorithm for ADRs, and nurse/clinician feedback. We reviewed encounters of patients seen in UC with a PAL monthly during baseline (October 2022-September 2023) and study periods (October 2023-September 2024). We tracked the percent of PALs in UC and the percent of patients delabeled as our outcome measures. We tracked the percent of PALs canceled by history alone and the percent of patients referred for antibiotic challenge testing (ACT) as our process measures. The percentage of patients relabeled with PALs served as our balancing measure. We surveyed UC nurses and clinicians on the impact the project had on their evaluation and management of PALs using a 5-point Likert scale.
Results: We reviewed 10,678 encounters. Although monthly PAL rates did not change (7.1%), the center line (CL) for delabeled patients had an upward shift from 2% to 4.5% in October 2023. Patients referred for ACT had an upward CL shift from 0.6% to 5.3%. PALs canceled had an upward CL shift from 1.8% to 3.9% (Figure 1). 383 (3.6%) patients were delabeled; 321 (86.9%) cancelled by history and 50 (13.1%) after ACT. Though none had hypersensitivity reactions, 3 delabeled patients (0.78%) had PALs reapplied. With attrition at each step in the scheduling process, only 28.6% of patients referred between October 2023-February 2024 completed their subspecialty appointments, with an average delay of 4 months between referral and subspecialty appointment (Figure 2). Pre/post project survey results showed increases in how often nurses and clinicians requested details about PALs (12.5% to 96.9%) and referred patients for further allergy evaluation when appropriate (0% to 68.8%) (Figure 3).
Conclusion(s): Though we did not decrease the overall percentage of PALs in our fluctuating UC population, we saw a positive impact on UC clinicians’ and nurses’ approach to patients with PALs. We demonstrated that many patients can safely have their PALs canceled by taking a thorough ADR history without ACT. Next steps include streamlining the referral and scheduling processes to reduce patient attrition.
Figure 1: Shewhart Control Charts for (A) Primary and (B) Secondary Outcome and (C) and (D) Process Measures
.jpg) Our primary outcome measure (A) was the percent of patients with penicillin allergy labels seen in the urgent care each month. Our secondary outcome measure (B) was the percent of patients who had their penicillin allergy delabeled. Process measures included (C) the percent of penicillin allergy labels canceled by history alone and (D) the percent of patients with penicillin allergy labels who were referred for subspecialty evaluation to either Allergy or Infectious Disease clinics.
Our primary outcome measure (A) was the percent of patients with penicillin allergy labels seen in the urgent care each month. Our secondary outcome measure (B) was the percent of patients who had their penicillin allergy delabeled. Process measures included (C) the percent of penicillin allergy labels canceled by history alone and (D) the percent of patients with penicillin allergy labels who were referred for subspecialty evaluation to either Allergy or Infectious Disease clinics. Abbreviations: UCL-Upper Control Limit, CL-Center Line, LCL-Lower Control Limit, PALs-Penicillin Allergy Labels, UC-Urgent Care, PCN-Penicillin, ADR-Adverse Drug Reaction, D/C-Discharge, Instructns-Instructions
Figure 2: Patient Attrition from Referral to Subspecialty Visit
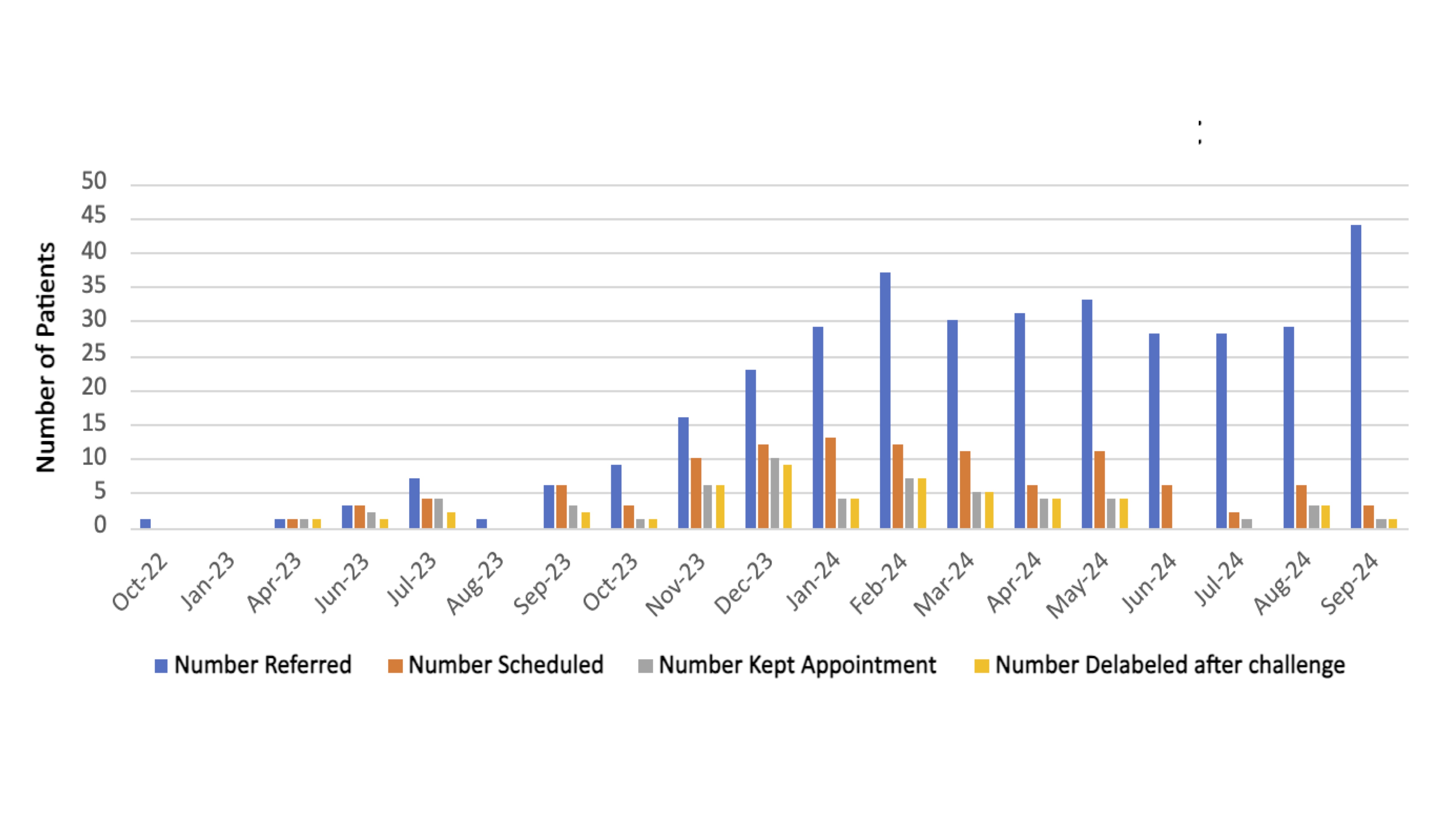 This bar graph illustrates the progressive patient attrition from referral to subspecialty visit with the number of patients referred for penicillin allergy evaluation depicted by the blue bars, the number of patients scheduled for subspecialty appointment depicted by the orange bars, and the number of patients who kept their subspecialty appointment depicted by the gray bars. Many patients could not be reached to schedule their subspecialty appointments. Many patients referred in June 2023 and later have not yet had their subspecialty appointments scheduled or been evaluated due to the 4-5 month delay between referral and subspecialty visit.
This bar graph illustrates the progressive patient attrition from referral to subspecialty visit with the number of patients referred for penicillin allergy evaluation depicted by the blue bars, the number of patients scheduled for subspecialty appointment depicted by the orange bars, and the number of patients who kept their subspecialty appointment depicted by the gray bars. Many patients could not be reached to schedule their subspecialty appointments. Many patients referred in June 2023 and later have not yet had their subspecialty appointments scheduled or been evaluated due to the 4-5 month delay between referral and subspecialty visit.Figure 3: Pre/Post Project Positive Survey Responses Regarding Penicillin Allergy Label Evaluation
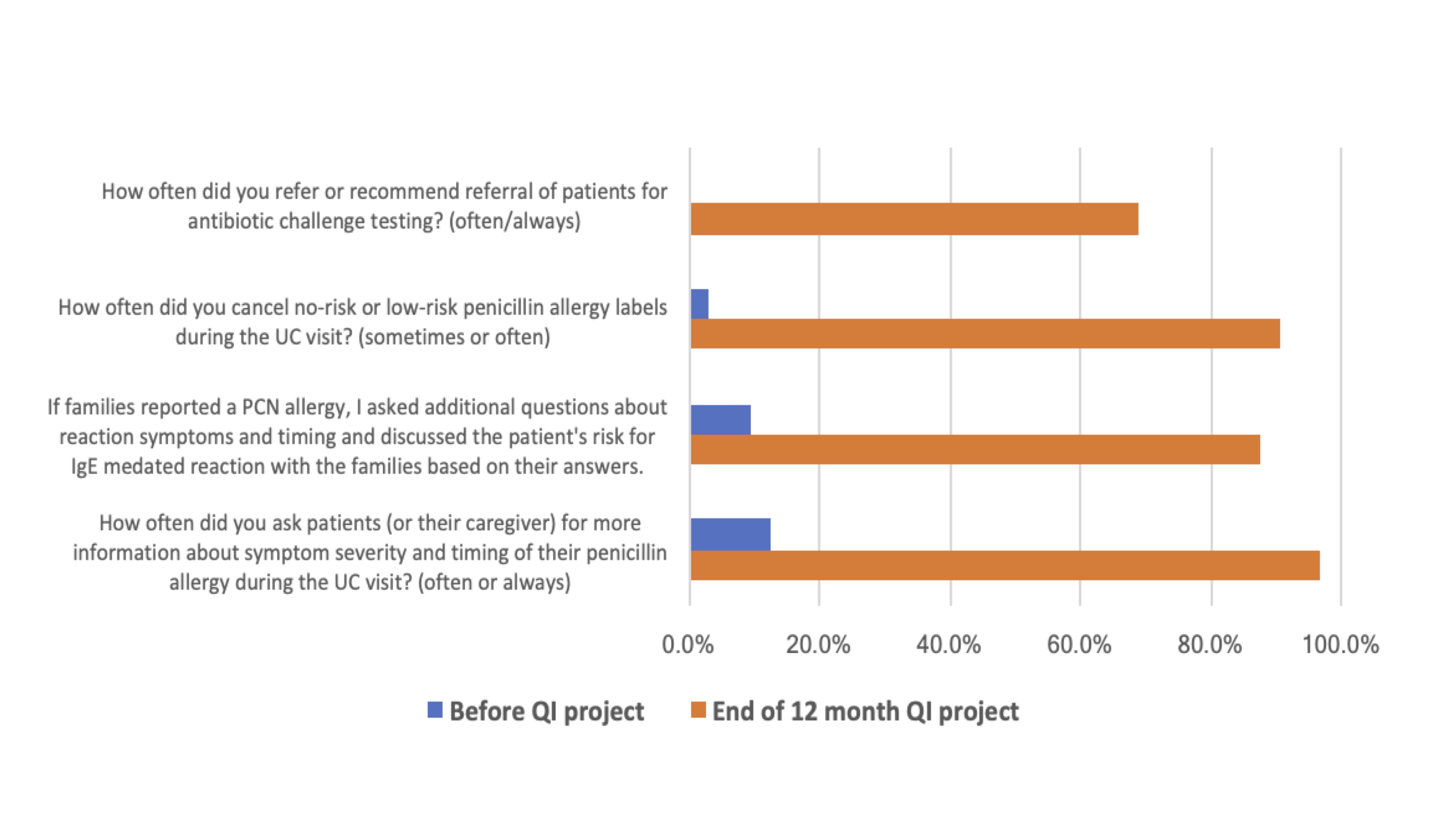 Nurse and clinician survey responses on their approach to evaluating penicillin allergy labels before and after the quality improvement project.
Nurse and clinician survey responses on their approach to evaluating penicillin allergy labels before and after the quality improvement project.Abbreviations: UC-Urgent Care, PCN-Penicillin, QI-Quality Improvement


