Health Equity/Social Determinants of Health 3
Session: Health Equity/Social Determinants of Health 3
434 - Promoting the recruitment of historically underrepresented children and families in clinical trials: Perspectives of pediatric clinic staff
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 434.6194
Grace W. Ryan, University of Massachusetts Medical School, Worcester, MA, United States; Janvi D. Nanavati, University of Massachusetts Medical School, Worcester, MA, United States; Daniel Mendoza Martinez, University of Massachusetts Medical School, Worcester, MA, United States; Melissa Goulding, University of Massachusetts Medical School, Worcester, MA, United States; Sonia Radu, University of Massachusetts Medical School, Uxbridge, MA, United States; Michelle Spano, UMass Chan Medical School, Worcester, MA, United States; Ted Kremer, UMass Memorial Children's Medical Center, Worcester, MA, United States; Kali Pereira, UMass Memorial Children's Medical Center, Worcester, MA, United States; John T. Almeida, University of Massachusetts Medical School, Springfield, MA, United States; Christine Frisard, University of Massachusetts Medical School, Worcester, MA, United States; Sybil Crawford, University of Massachusetts Medical School, Worcester, MA, United States; Milagros C.. Rosal, UMass Chan Medical School, Worcester, MA, United States; Nancy Byatt, UMass Chan Medical School/UMass Memorial Health, Shrewsbury, MA, United States; Stephenie C. Lemon, UMass Chan Medical School, Worcester, MA, United States; Lori Pbert, University of Massachusetts Medical School, Harvard, MA, United States; Michelle Trivedi, UMass Memorial Children's Medical Center, Worcester, MA, United States

Grace Ryan, PhD, MPH (she/her/hers)
Assistant Professor
University of Massachusetts Medical School
Worcester, Massachusetts, United States
Presenting Author(s)
Background: Despite the importance of diversity in pediatric clinical trials, participation from children and families from underrepresented minority (URM) backgrounds remains low. While barriers to adult URM participation have been studied, little is known about strategies that can be used in pediatric practice settings to address these issues and improve representation of URM families in research.
Objective: We sought to qualitatively explore perspectives of pediatric clinic staff on barriers and facilitators to recruitment of URM children and families into clinical trials.
Design/Methods: We conducted semi-structured interviews with pediatric clinic staff who supported the recruitment of URM children and families into a clinical trial. We designed an interview guide to elicit perspectives on the barriers and facilitators to recruitment, with a focus on experiences that facilitated or hindered the recruitment of URM children and families. We used rapid template analysis and identified themes and sub-themes within the data along with representative quotes.
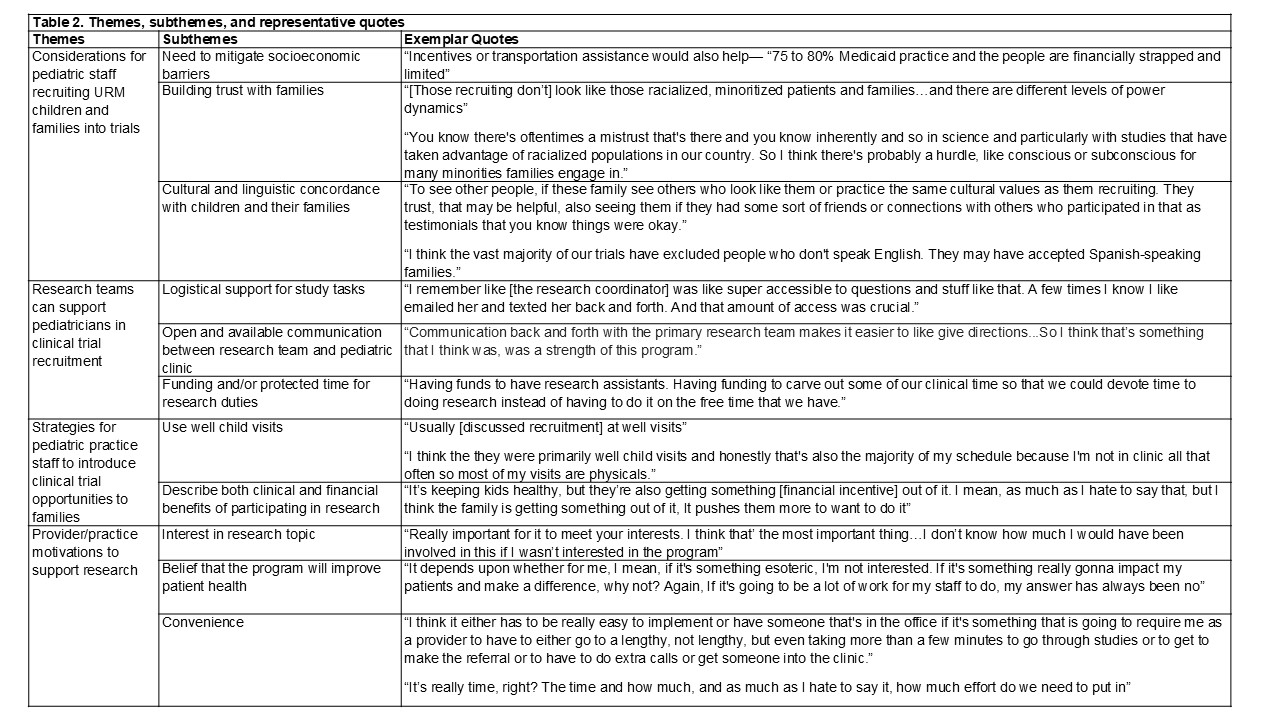
Results: Of 29 participants invited, we conducted interviews with 20 pediatric clinic staff from both academic medical center and community-based practices; interviews averaged 15 minutes (range: 8-28 minutes). We identified four themes: (1) Considerations for pediatric clinic staff recruiting URM children and families into trials, (2) Support research teams can provide pediatricians in clinical trial recruitment, (3) Strategies for pediatric practice staff to introduce clinical trial opportunities to families, and (4) Provider/practice motivations to support clinical trial recruitment.
Conclusion(s): We identified specific strategies to increase representation of URM children and families in trials. Research teams can employ diverse study staff that have similar racial/ethnic and cultural backgrounds to the participants they strive to include, ensure study materials are inclusive of non-English speakers, partner with clinics that employ staff who can communicate with patients in their preferred language, and prioritize ongoing communication with clinics to support issues that arise during recruitment. Pediatricians can use well child visits to present research opportunities and ensure they explain potential clinical and financial benefits of participating and acknowledge potential mistrust patients may have in research. Integrating these actionable steps has potential to improve URM participation in pediatric clinical trials, ultimately facilitating research that better addresses widespread child health inequities.
Table 1. Participant Characteristics: Pediatric Providers and Clinic Staff (n=20)
.jpg)
Table 2. Themes, subthemes, and representative quotes