Neonatal GI Physiology & NEC 2
Session: Neonatal GI Physiology & NEC 2
719 - Effects of Prophylactic Probiotics Supplementation on Infants Born Very Preterm or Very Low Birth Weight at a Community Hospital
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 719.4800
Sameera Chiruvolu, Case Western Reserve University, Cleveland, OH, United States; Amy Reedy, Baylor Scott & White Medical Center of McKinney, McKinney, TX, United States; Jordan Reis, Texas A&M Health Science Center College of Medicine, Dallas, TX, United States

Sameera Chiruvolu (she/her/hers)
Undergraduate student
Case Western Reserve University
Cleveland, Ohio, United States
Presenting Author(s)
Background: Intestinal dysbiosis appears to be an important risk factor for the development of necrotizing enterocolitis (NEC) in very preterm (VP) infants. Therefore, optimizing the gut microbiome with probiotics may be a favorable strategy to prevent NEC. In the United States, the use of probiotics in VP infants had been increasing until the recent FDA warning which prompted majority of the hospitals, including ours, to pause administration. We feel it is important to report our experience with routine probiotics administration and continue to analyze the effects of pausing probiotics in the community hospital setting.
Objective: To evaluate the effects of a multi-strain NICU-specific probiotic product (Similac Triblend™ consisting of Bifidobacterium lactis (BB-12®), Bifidobacterium infantis (BB-02™), and Streptococcus thermophilis (TH-4®)) on infants born VP or very low birth weight (VLBW) in a suburban community hospital in North Texas. We hypothesized that a guideline-driven routine supplementation would be associated with a reduction of NEC incidence from the baseline of 10% (one year prior) to 5% in two years after implementation.
Design/Methods: In this cohort study, 60 infants born VP or VLBW who received probiotics during the prospective study period (two years after implementation; October 1, 2020 to September 30, 2022; Probiotics group) were compared to a retrospective cohort of 34 VP or VLBW infants who qualified to receive probiotics, but did not, as they were born one year prior to implementation (October 1, 2019 to September 30, 2020; No probiotics group). The outcomes of interest were NEC, death and late-onset sepsis.
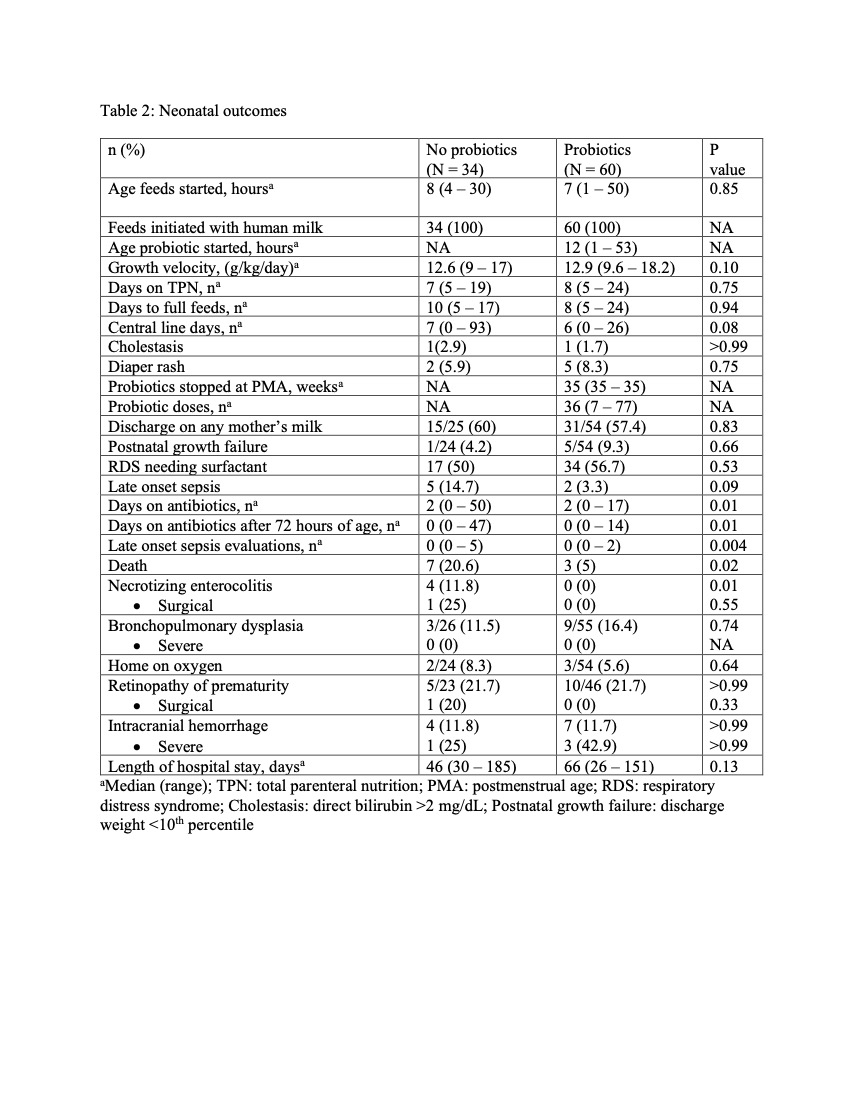
Results: There were no differences in maternal or neonatal characteristics between both groups (Table 1). Median age of starting probiotics was 12 hours of life and were given until 35 weeks post menstrual age. The growth velocity and the incidence of postnatal growth failure were similar between both groups. The incidence of NEC was significantly lower in the Probiotics group (0%) compared to 11.8% in the No probiotics group. Similarly, the incidence of death significantly decreased in the Probiotics group (5%) compared to 20.6% in the No probiotics group (Table 2). No cases of probiotic sepsis were observed.
Conclusion(s): After implementation of guideline-driven routine supplementation of probiotics in infants born VP or VLBW at a community hospital, significant reduction was noted in the incidence of NEC and death with no adverse effects. We are in the process of analyzing the effects of pausing the routine probiotics administration in this population.
Table 1: Maternal and neonatal characteristics
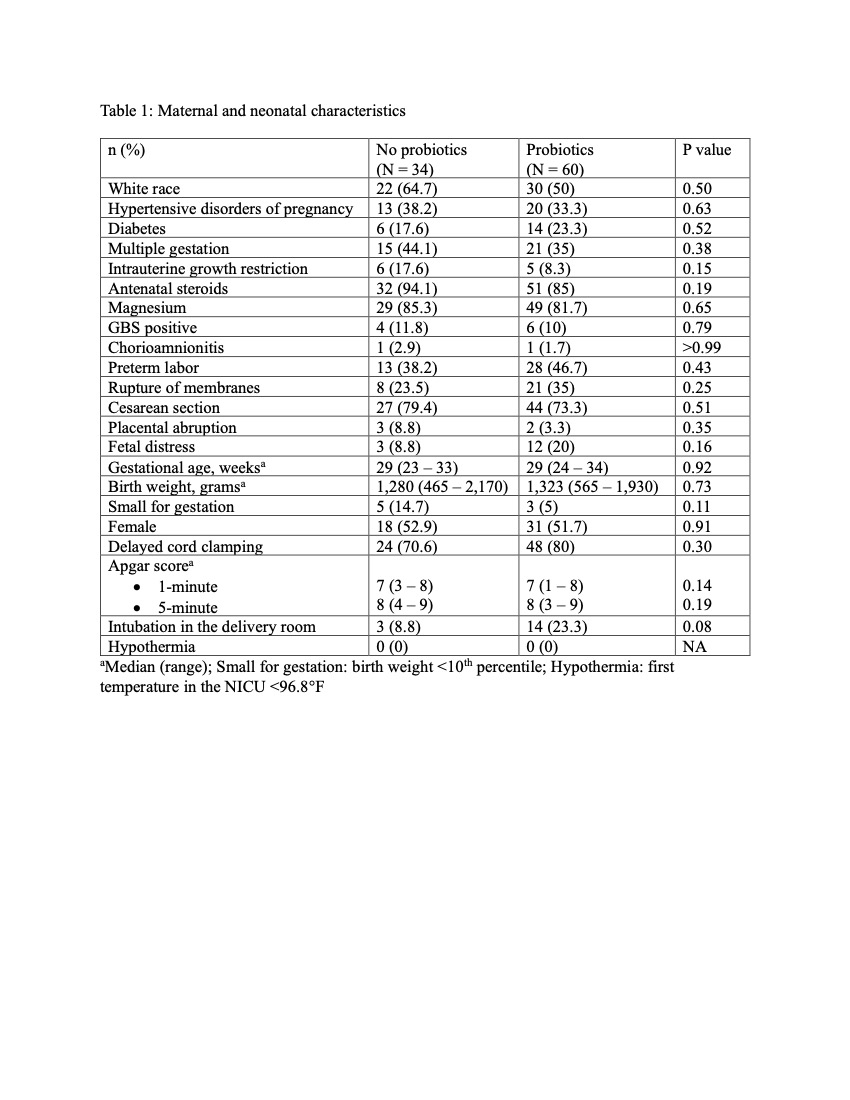
Table 2: Neonatal outcomes