Neonatal General 9: Hematology, Bilirubin and Feeding
Session: Neonatal General 9: Hematology, Bilirubin and Feeding
694 - Post ECMO utilization of Cell Saver blood from the ECMO circuit for blood transfusions within 8 hours of decannulation in Neonates.
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 694.6497
Lindsey Tyson, UVA Childrens, Palmyra, VA, United States; Ross Thomas, UVA Childrens, Ruckersville, VA, United States; Brian Clouse, UVA Childrens, Charlottesville, VA, United States; Samuel Addison, University of Virginia School of Medicine, Crozet, VA, United States; David A. Kaufman, University of Virginia School of Medicine, Charlottesville, VA, United States

David A. Kaufman, MD
Professor/Attending
University of Virginia School of Medicine
Charlottesville, Virginia, United States
Presenting Author(s)
Background: Cell saver technology now allows us to utilize the left over blood from the ECMO Circuit around decannulation and up to 8 hours from collection of the blood. There is about 300 ml in a neonatal ECMO circuit that can be spun in the cell saver in ~15 minutes. This blood can be transfused back to the patient to reduce post ECMO anemia, blood exposure, healthcare costs and Improve blood availability for other patients.
Cell saver blood differs from PRBCs in several ways: 1) doesn't undergo the same adverse effects of storage as banked PRBCs, 2) retains its elliptical profile and deformability better than stored allogeneic red cells, and 3) has increased concentrations of 2,3-diphosphoglycerate and ATP, which can improve tissue oxygen delivery. Cell saver blood can reduce the risk of transfusion-related infectious diseases and RBC alloimmunization for certain populations, which are associated with allogeneic transfusions.
Furthermore, cell saver blood once processed is at the bedside and can be given promptly save blood bank personnel time.
Objective: Evaluate the volume and hematocrit of cell saver blood processed from the neonatal ECMO circuit and percent of patients who receive it post ECMO.
Design/Methods: SInce 2017, cell saver blood was processed post ECMO from the ECMO circuit using the Autotransfusion system (LivaNova®) using a 125 ml bowl. During decannulation, the ECMO circuit tubing is looped together to allow recirculation of the blood to prevent clotting within the system. The the cell saver tubing is then connected to the ECMO circuit to begin processing. The cell saver was not used for any circuit that clotted and when a patient had life support discontinued for redirection of care.
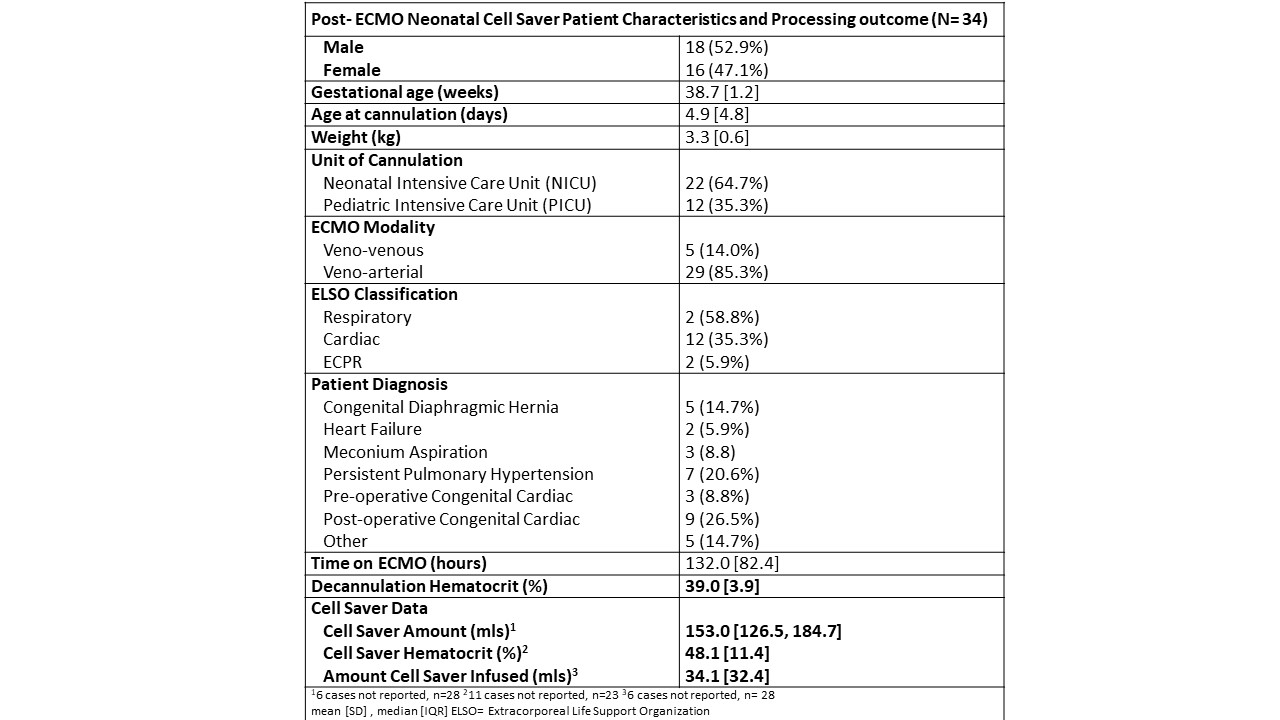
Results: From 2017 to 2024, 34 pediatric patients cared for in the NICU and PICU had their ECMO circuit processed with the cell saver. Patient demographics and cell saver data are summarized in Table 1. Cell saver yielded a median (interquartile range) of 153 ml (126.5, 184.7) with a mean (±SD) hematocrit of 48.1 (±11.4). Cell saver blood was administered in 20 of these 34 patients with no adverse events.
Conclusion(s): Patients can safely receive a portion of their red blood cells and blood after ECMO is discontinued, potentially preventing anemia, hypotension, and another allogenic blood exposure as well as with substantial cost and blood bank time savings. Cell salvage equipment is easy to operate, inexpensive (estimate $93) and cheaper than a transfusion from blood bank (estimate in the United States: ~$550).
Post- ECMO Neonatal Cell Saver Patient Characteristics and Processing outcome (N= 34)