Developmental and Behavioral Pediatrics 4: ADHD
Session: Developmental and Behavioral Pediatrics 4: ADHD
798 - Cyproheptadine for Managing Psychostimulant-Induced Weight Loss in Pediatric and Adolescent ADHD Patients: A Retrospective Chart Review
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 798.4273
Khaled Mohammed, Mayo Clinic Children's Center, Rochester, MN, United States; Dima Bechenati, Mayo Clinic Children's Center, Rochester, MN, United States; Renee M. Breland, Mayo Clinic Children's Center, Cannon Falls, MN, United States; Jyoti Bhagia, Mayo Clinic Children's Center, Rochester, MN, United States; Tamim Rajjo, Mayo Clinic Alix School of Medicine, Rochester, MN, United States
- KM
Khaled Mohammed, MD
Assistant Professor
Mayo Clinic Children's
Rochester, Minnesota, United States
Presenting Author(s)
Background: Attention-Deficit/Hyperactivity Disorder (ADHD) affects 7 million U.S. children ages 3-17, making it the most prevalent neurobehavioral disorder. While stimulants are effective in managing symptoms, they often cause side effects, including appetite suppression and weight loss. Cyproheptadine, a first-generation antihistamine, has been used off-label for appetite stimulation and weight gain in malnourished and/or underweight patients with various health conditions. While studies suggest cyproheptadine is safe, well-tolerated, and effective for weight gain in various populations, research on its use for stimulant-induced weight loss in children and adolescents with ADHD remains limited.
Objective: This study aims to evaluate the effects of cyproheptadine on weight and BMI in pediatric and adolescents patients with ADHD experiencing stimulant-induced weight loss.
Design/Methods: A retrospective chart review was conducted for pediatric and adolescent ADHD patients attending Mayo Clinic primary care or psychiatric offices from 2018 to 2023. Eligible patients, aged 4-21 years, had an ADHD diagnosis identified by ICD-10 codes and were on psychostimulant medication with documented weight loss or decreased appetite. Patients with chronic illnesses affecting weight (e.g., endocrine or gastrointestinal disorders, cancers) or on medications causing weight loss or appetite suppression were excluded. Data were collected in Excel and analyzed in SPSS v29.
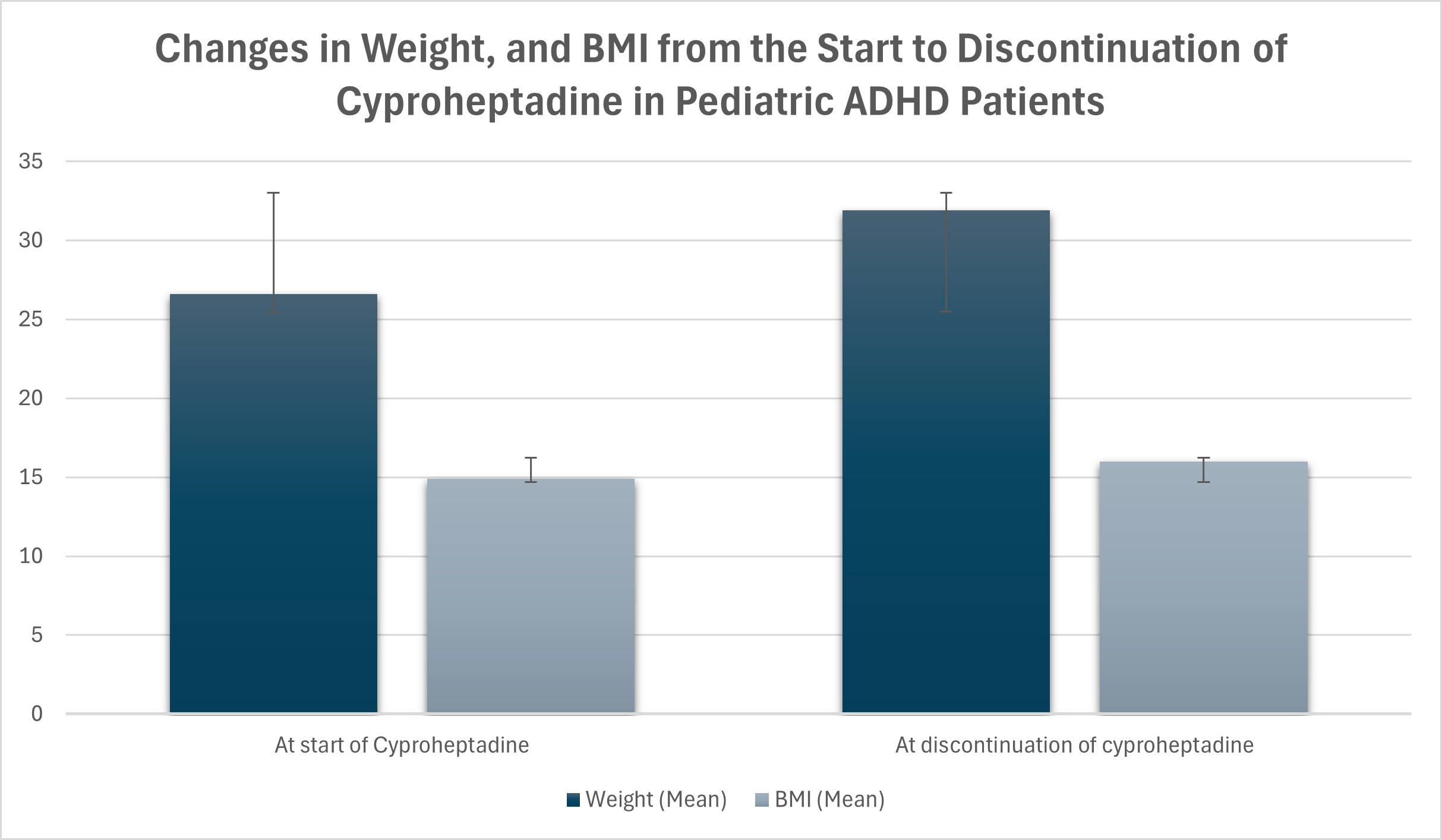
Results: A total of 107 patients met our inclusion criteria. Mean age was 13.06 ± 3.4 years old. The cohort were predominantly males (71%) and White (85%). The most prevalant ADHD subtype was combined type (71.4%). Common comorbidities included anxiety (19.6%), autism spectrum disorder (17.8%), and depression (8.4%). Mean follow up from cyproheptadine initiation to discontinuation was 65.9 ± 8.8 weeks. There was a significant increase in weight over time after starting cyproheptadine with mean weight increase 5.14 kg (SD = 4.91) (95% CI: 3.94–6.35, p < 0.001) from the start to discontinuation of Cyproheptadine, with a corresponding increase in BMI of 1.19 units (95% CI: 0.86–1.52, p < 0.001). Additionally, weight velocity improved significantly, with mean increase of 0.0156 kg/day (SD = 0.0206, p < 0.001).
Conclusion(s): Our findings suggest that cyproheptadine is an effective intervention in mitigating stimulant-induced weight loss in pediatric and adolescent ADHD patients. Future prospective and randomized studies are recommended to explore long-term safety and efficacy of cyproheptadine in this patient population.
Table 1: Baseline charteristics of included patients:
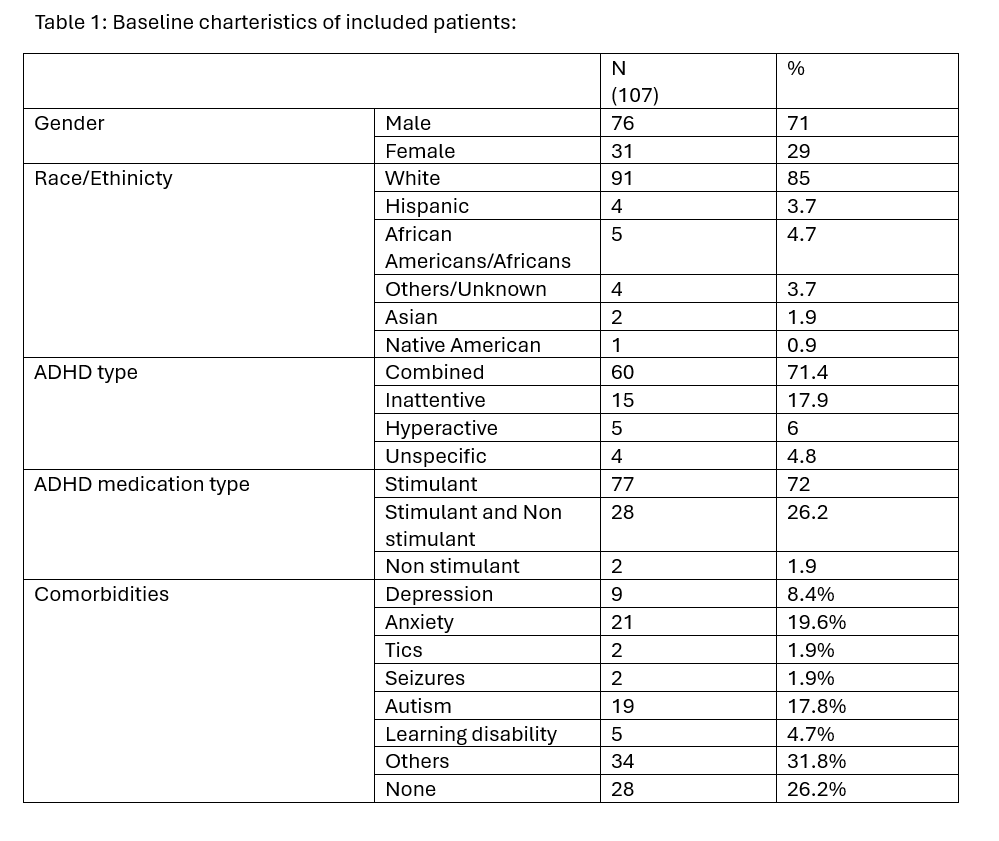
Figure 1: Changes in Weight, and BMI from the Start to Discontinuation of Cyproheptadine in Pediatric ADHD Patients