Neonatal Fetal Nutrition & Metabolism 3
Session: Neonatal Fetal Nutrition & Metabolism 3
629 - Growing for Good - Comparing Growth and Other Outcomes Pre and Post Implementation of an Exclusive Human Milk Diet
Monday, April 28, 2025
7:00am - 9:15am HST
Publication Number: 629.5447
Sharanah G. Ridore, John R. Oishei Children's Hospital, Buffalo, NY, United States; Cara L. McLaughlin, Oishei Children's Hospital, Buffalo, NY, United States; Deepali Handa, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; James Shelton, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; Nicolette Sarvis, John R. Oishei Children's Hospital, Buffalo, NY, United States; Kelsey Voelker, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; Valerie Elberson, John R. Oishei Children's Hospital, Buffalo, NY, United States; Brian Wrotniak, John R. Oishei Children's Hospital, Buffalo, NY, United States; Anne Marie Reynolds, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; Kavya Rao, Oishei Childrens Hospital - University at Buffalo, Buffalo, NY, United States

Sharanah G. Ridore, D.O. (she/her/hers)
Fellow
John R. Oishei Children's Hospital
Buffalo, New York, United States
Presenting Author(s)
Background: Feeding an exclusive human milk diet (EHMD) in premature infants with very low birth weights ( < 1500g) compared with a traditional diet that uses bovine milk-based fortifiers (BMBF) added to Mother’s Own Milk (MOM) or Donor Human Milk (DHM) has shown beneficial results in reducing morbidities associated with prematurity. There are some concerns associated with DHM feeding, including deactivation of many of the biologically active components, elimination of potentially beneficial bacteria by pasteurization, and suboptimal infant growth rates when compared to formula. Additionally, barriers such as the lack of standardized feeding protocols, variability in practice, and limited long-term growth outcome data with the use of EHMD in the neonatal intensive care unit (NICU), pose a conundrum between the use of BMBF versus EHMD.
Objective: To compare growth and additional secondary outcomes in preterm infants who received MOM/DHM fortified with BMBF (Epoch-1) with those who received an EHMD fortified with human milk-based fortifier (HMBF)(Epoch-2).
Design/Methods: Single-center, retrospective cohort study that assessed growth and additional secondary outcomes in infants born at the Level IV NICU at John Oishei Children's Hospital, between August 2021 to December 2023 with gestational age < 34 weeks and birth weight < 1250 g before and after the implementation of a standardized feeding protocol for EHMD.
Results: Study included 200 infants < 34 weeks GA with birth weights between 520 g and 1250 g. At birth, there were no differences in BW, HC, and length (Table 1). Compared with infants in the BMBF group, infants in the EHMD group had increased growth velocities from birth to 34 weeks PMA (12.36±7.26 vs 10.5±7.12 g/kg/d; p = 0.135) with no change in HC and height after reaching full enteral nutrition of 140 mL/kg/d by 34 weeks PMA (Table 2). Infants in the EHMD group had increased length change z scores (-1.55±1 vs 0.92±1 g/kg/d; p = 0.011). After the initiation of nutritional support, growth increased with the values of weight, HC, and height at 34 weeks PMA being similar in both groups. Compared with infants in the BMBF group, infants in the EHMD group had decreased rates of NEC ≥ Stage 2 (1.75% vs 12.8%; p = 0.002). There was no difference in days on TPN, time to full feeds, ventilator days, and/or mortality.
Conclusion(s): Implementing the standardized feeding protocol, which included earlier fortification with HMBF to MOM/DHM, was associated with a trend towards improved growth parameters in preterm infants. Future directions aimed at decreasing time on trophic feeds may be implicated to promote better growth.
Table 1. Comparison of Neonatal Anthropometric Data at Birth in Epochs 1 and 2.
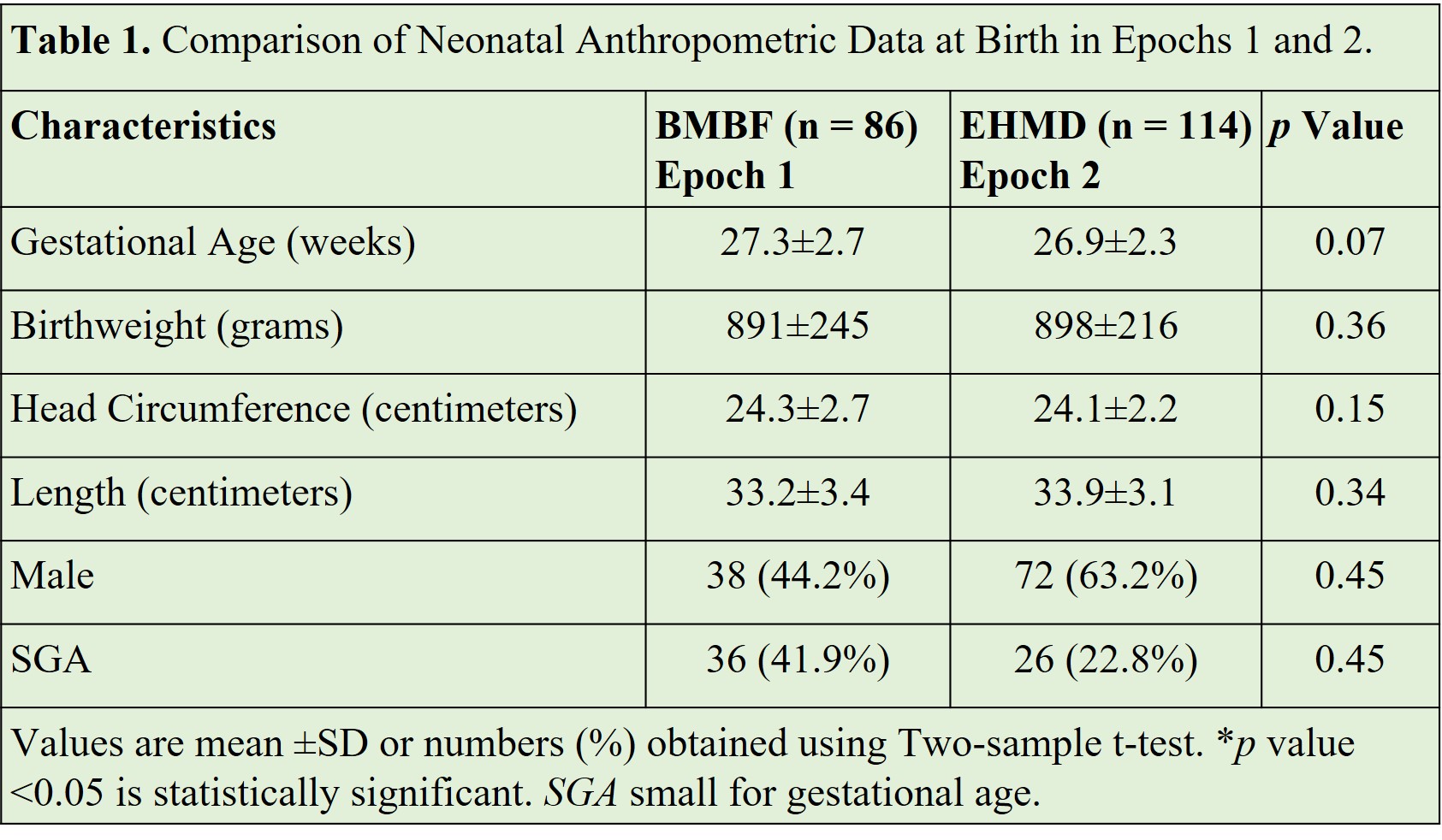
Table 2. Comparison of Growth Outcomes in Epochs 1 and 2 from Birth to 34 weeks PMA.
.jpg)
Table 3. Comparison of Clinical Outcomes in Epochs 1 and 2.
.jpg)

