Neonatal GI Physiology & NEC 1
Session: Neonatal GI Physiology & NEC 1
712 - Disease-Specific HNF4A Signature Persists in Long-Term Culture of Necrotizing Enterocolitis Patient-Derived Enteroids
Saturday, April 26, 2025
2:30pm - 4:45pm HST
Publication Number: 712.4293
Kathryn Burge, University of Oklahoma College of Medicine, Oklahoma City, OK, United States; Hala Chaaban, Oklahoma University health sciences, Okl, OK, United States; Adam Wilson, Oklahoma Childrens Hospital at OU Health, Oklahoma City, OK, United States; Hua Zhong, University of Oklahoma College of Medicine, Oklahoma city, OK, United States; Karni S. Moshal, University of Oklahoma College of Medicine, Oklahoma City, OK, United States; Addison Franca, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States

Kathryn Y. Burge, PhD (she/her/hers)
Assistant Professor
University of Oklahoma Health Sciences Center
Oklahoma City, Oklahoma, United States
Presenting Author(s)
Background: Patient-derived enteroids, 3D ex vivo intestinal epithelial cultures, have advanced translational research. While enteroids sourced from adults are generally well-characterized, those from preterm infants are far less so given inherent scarcity of available tissues. The degree to which enteroids retain physiological features of their source tissue rather than converging on a common phenotype dictated by tissue culture media has been widely debated. Necrotizing enterocolitis (NEC), a devastating preterm intestinal inflammatory disease, is associated with altered epithelial differentiation and stem cell proliferation, features likely to influence enteroid cultures. As a therapeutically intractable disease, NEC treatment is focused on disease prevention. Therefore, inflammatory naïve enteroid cultures may be preferable to those retaining proliferative deficiencies in experiments evaluating prophylactics.
Objective: Determine the degree to which enteroids from heterogeneous origins converge on culture media-driven phenotypes rather than retain disease-specific signatures.
Design/Methods: Enteroids derived from the ileum of a preterm infant with NEC (n = 1) and intestinal atresia (IA, n = 1) were cultured, differentiated, live cell sorted, and submitted for single-cell RNA-sequencing using the 10X Genomics Chromium platform. Sequencing data was filtered, annotated, and UMAPs were plotted to establish heterogeneity in cell types. Cell-type-specific comparisons were uploaded to Ingenuity Pathway Analysis for functional pathway enrichment analyses.
Results: Compared with IA enteroids, NEC enteroids contained more cycling and absorptive TA (transit-amplifying) and bestrophin 4 (BEST4+) cells and fewer stressed TA, stem, secretory TA, and enterocytes, indicating a shift toward differentiated and absorptive cell types (Fig. 1). IPA analysis predicted highly significant and downregulated hepatocyte nuclear factor 4A (HNF4A) activity in all NEC enteroid cell types outside of secretory progenitor and BEST4+ cells. Interestingly, HNF4A mediates epigenetic modifications in surgical NEC, and suppressed HNF4A activity is associated with inflammatory cytokine signaling and disrupted brush borders.
Conclusion(s): Important regulatory characteristics persist in enteroid culture long-term. With roles in cell proliferation, apoptosis, stem cell renewal, and differentiation, persistence of HNF4A source tissue signatures represents a significant experimental variable for studies attempting to evaluate disease-preventing therapies, even in the absence of the microbiome.
NEC enteroids display altered proliferation and differentiation of epithelial cell types.
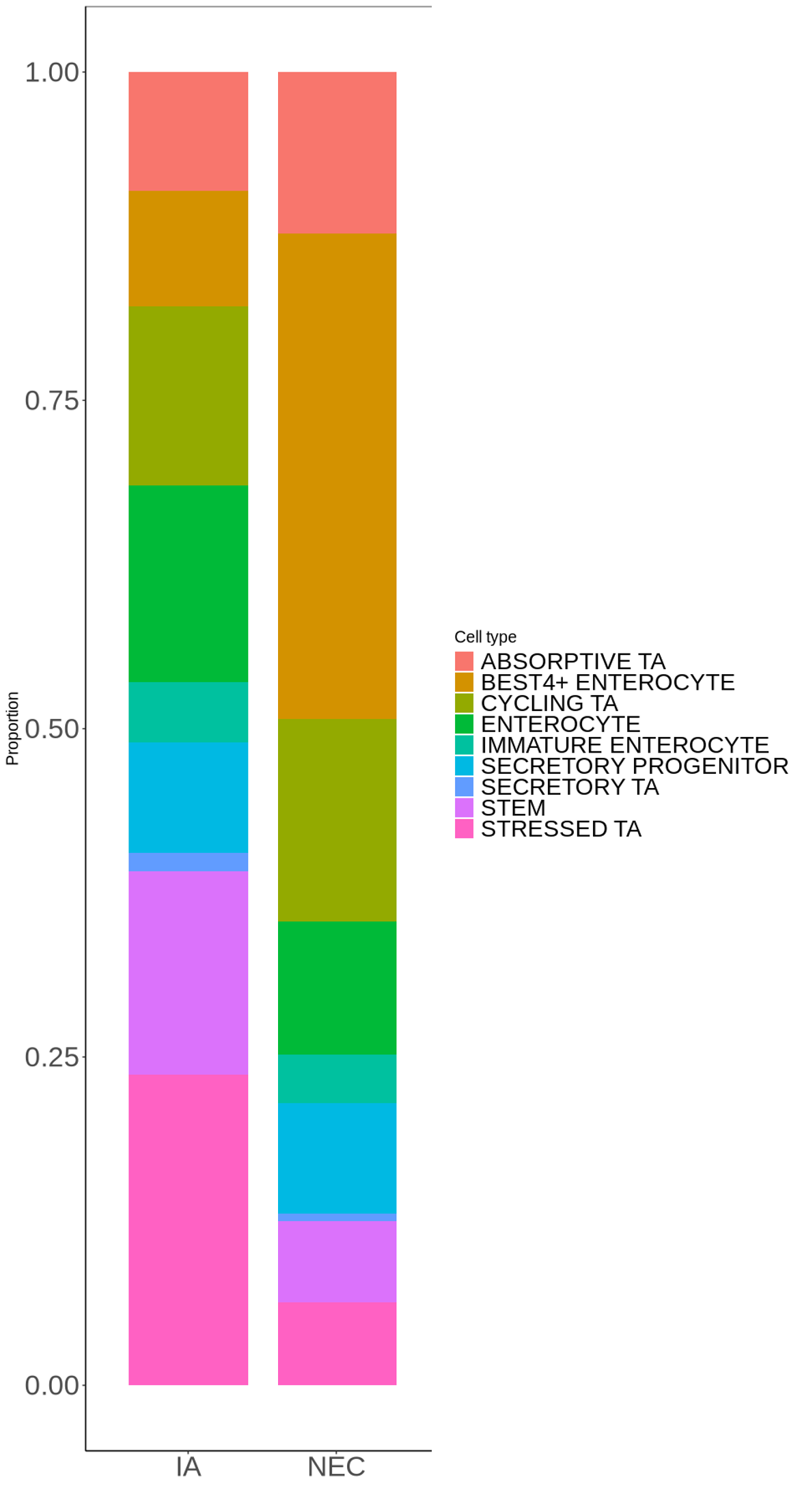 Average proportion of each epithelial cell type in enteroids sourced from preterm intestinal atresia (IA) and necrotizing enterocolitis (NEC) ileum.
Average proportion of each epithelial cell type in enteroids sourced from preterm intestinal atresia (IA) and necrotizing enterocolitis (NEC) ileum.
