Emergency Medicine 9
Session: Emergency Medicine 9
132 - Prehospital Use of Levetiracetam in Pediatric Status Epilepticus
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 132.6423
Alexandra Licona-Freudenstein, University of Washington/ Seattle Children's Hospital, Seattle, WA, United States; Lindsey Morgan, Seattle Children’s Hospital, Seattle, WA, United States; Andrew McCoy, Univeristy of Washington, Snohomish, WA, United States; David L. Murphy, Univeristy of Washington, Seattle, WA, United States; Jessica Jeanne. Wall, University of Washington School of Medicine, Seattle, WA, United States

Alexandra Licona-Freudenstein, MD (she/her/hers)
Fellow
University of Washington/ Seattle Children's Hospital
Seattle, Washington, United States
Presenting Author(s)
Background: Seizures are 6-10% of prehospital pediatric medical emergencies. 30-40% of seizures are benzodiazepine-resistant and require escalation to a second-line anti-seizure medication (ASM). A recent review of 26941 prehospital pediatric patients revealed only 13 received levetiracetam (LEV). There are no pediatric studies to date evaluating prehospital LEV use. In October 2020, King County Advanced Life Support (ALS) units began carrying LEV.
Objective: We aim to describe the impact of LEV availability in the prehospital setting.
Design/Methods: Retrospective cohort study including patients 1 month to 18 years treated for seizure by our local ALS agency during 1/2017 – 12/2023 (baseline 1/2017-9/2020; intervention 10/2020-12/2023). Charts were reviewed for medication administration and included if medications were given for indication of seizure. Patients that met the inclusion criteria and were transported to Seattle Children’s Hospital (SCH) were identified for analysis. Electronic medical records were reviewed for intubation and disposition. Children with non-convulsive seizure onset, psychogenic non-epileptic seizure, and children not transported to SCH were excluded.
Results: There were 344 patients, 154 receiving BZD in baseline period (pre-LEV), 149 receiving BZD only in intervention period (post-LEV) and 41 receiving BZD+LEV. Continuous seizure was the reported indication for 86/154 (56%) of the pre-LEV group, 87/149 (58%) of the post-LEV BZD only group and 19/41 (46%) of the BZD+LEV group. Cluster seizures were the reported indication in 36/154 (23%) of the pre-LEV group, 40/149 (27%) of the post-LEV BZD only group and 19/41 (46%) of the BZD-LEV group. Prehospital intubation occurred in 19/154 (12%) of the pre-LEV group, 6/149 (4.0%) of the post-LEV BZD only group and 6/41 (15%) of the BZD+LEV group. Of patients receiving BZD+LEV, 24/41 (59%) were categorized as complex chronic, with 7/41 (17%) non-complex chronic and 10/41 (24%) non-chronic. 2/41 (4.9%) patients treated with BZD+LEV were Black while 29/149 (19%) Black patients received post-LEV BZD only and 17/154 (11%) were treated pre-LEV. 14/41 (34%) of BZD+LEV patients were discharged home from the Emergency Department.
Conclusion(s): King County ALS units are one of the first to carry levetiracetam for use in pediatric status epilepticus. Descriptive analysis of initial use suggests feasibility and safety. Analysis is ongoing for efficacy, timing, and identification of any inequities for a future intervention focus.
Demographic Information and Outcomes
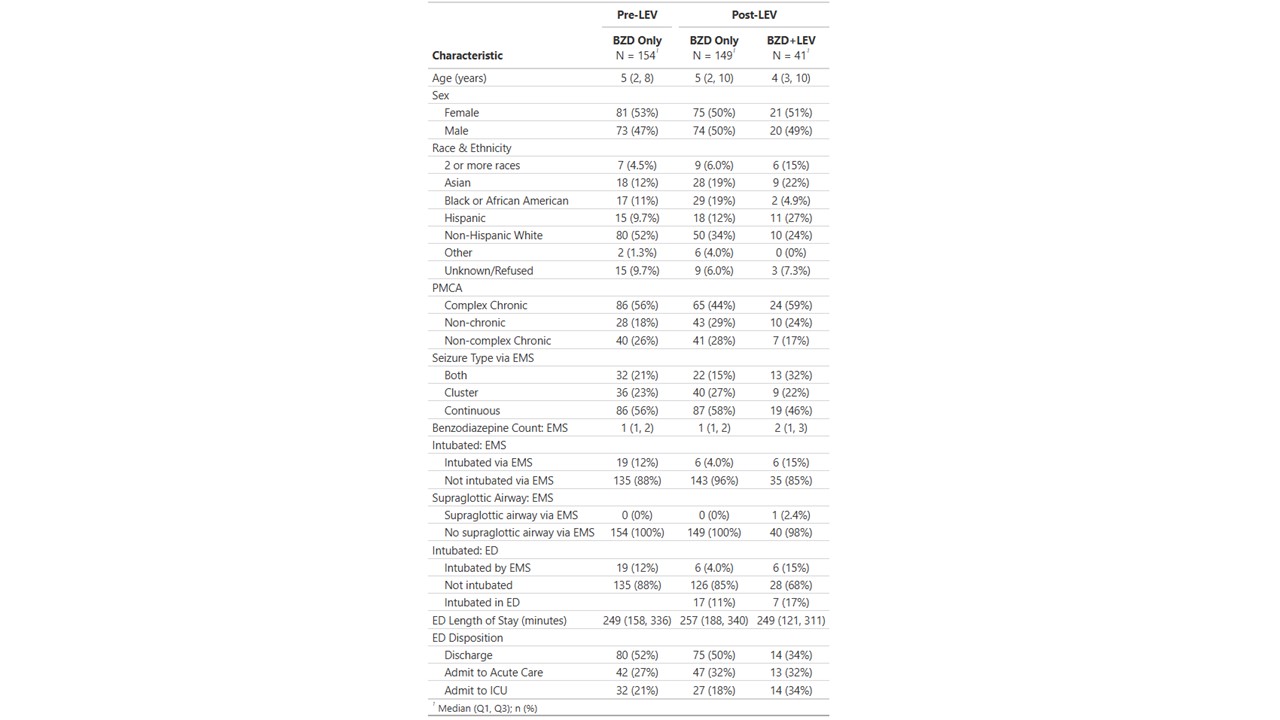 Patients are divided into 3 categories. Pre-LEV: received benzodiazepines only during baseline timeframe; post-LEV BZD only: received benzodiazepines only during intervention phase; post-LEV BZD+LEV: received benzodiazepine and levetiracetam during intervention phase
Patients are divided into 3 categories. Pre-LEV: received benzodiazepines only during baseline timeframe; post-LEV BZD only: received benzodiazepines only during intervention phase; post-LEV BZD+LEV: received benzodiazepine and levetiracetam during intervention phaseBZD: benzodiazepine; LEV: levetiracetam; PMCA: pediatric medical complexity algorithm; EMS: emergency medical services; ED: emergency department; ICU: intensive care unit

