General Pediatrics 4
Session: General Pediatrics 4
229 - Adverse Events after (P)athogen-(Re)duced (P)latelets in (C)hildren in the United States (US) (PRePCUS)
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 229.3980
Chrysanne Bennett, Meharry Medical College, Brentwood, TN, United States; Jennifer Andrews, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States

Chrysanne Bennett
Medical Student
Meharry Medical College
Brentwood, Tennessee, United States
Presenting Author(s)
Background: Though transfusions are critical lifesaving therapies, transfusion reactions can lead to serious morbidity and even fatality. The risk of transfusion-associated bacterial sepsis is more commonly seen following platelet transfusions. Pathogen-reduced (PR) platelets are processed with amotosalen and exposure to UVA light, which impairs bacterial DNA and RNA replication, destroying the infectious capability of bacteria and other infectious organisms. Though PR platelets were approved by the Food and Drug Administration in 2014, they are not commonly used in the US.
Objective: The PRePCUS study aimed to collect data to address the safety and efficacy of PR platelets in a large sample of pediatric patients in the US, expanding upon the largest comparable study.
Design/Methods: A retrospective chart review was performed after Institutional Research Board exemption was obtained, gathering information about patients who received platelet transfusions at Monroe Carrell Jr Children’s Hospital between January 1, 2018, and July 1, 2024, during which time PR platelets were almost 100% of the platelet inventory dispensed. The frequency and types of transfusion reactions following transfusions of PR platelets were gathered and compared to those reported in the literature following conventional platelets. The frequency of red blood cell (RBC) transfusions administered in the 48-hours following an initial platelet transfusion, as a proxy marker for clinically significant bleeding, was compared to those reported in the literature following conventional platelets.
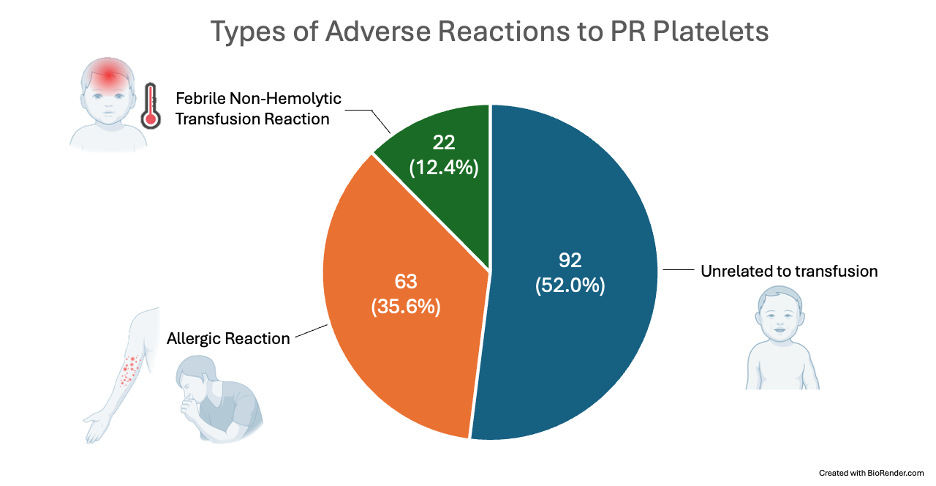
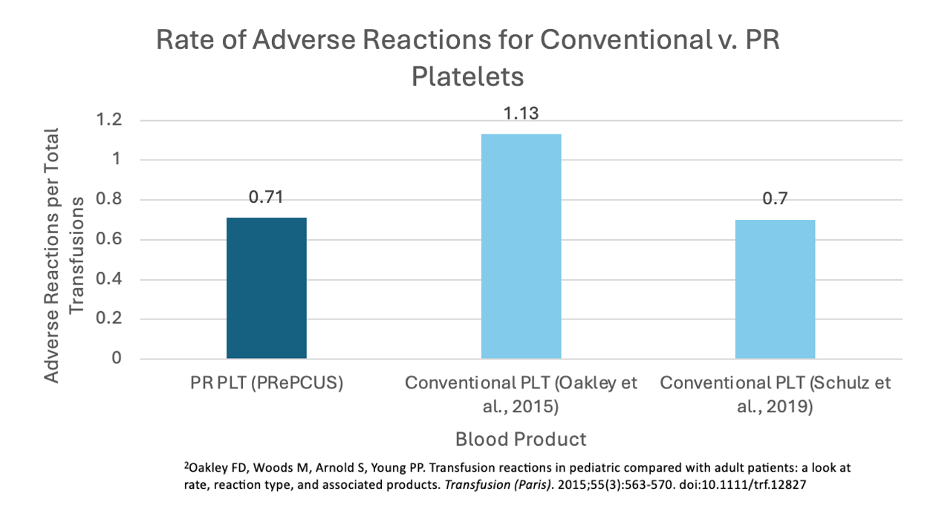
Results: A total of 3,206 pediatric patients received 24,791 PR platelet transfusions. A total of 177 suspected transfusion reactions were reported (0.71%), similar to reported reactions following conventional platelets (0.7%¹ to 1.13%²). A mean of 1.25 and 0.76 RBCs were utilized following PR platelet transfusion to infants aged 0-12 months and children aged 1-18 years, respectively, which is similar to 1.0 ± 1.3, 1.8 ± 2.2 , and 0.5 ± 0.9 RBCs utilized following transfusion of conventional platelets to NICU patients, non-NICU infants aged 0-12 months, and children aged 1-18 years, respectively¹. No cases of transfusion-associated bacterial sepsis were reported.
Conclusion(s): In summary, PRePCUS found transfusion reaction frequency and RBC utilization following PR platelet transfusions in over 3,000 children in the US similar to those reported after conventional platelets. Pediatricians in the US should consider adoption of PR platelets given the much lower risk of transfusion-transmitted infections, including fatal bacterial sepsis.
Number of PR Platelet Transfusions Recorded in PRePCUS v. the Previous Largest Study in the United States
.png)
Rates of Adverse Reactions for Conventional v. PR Platelets
Types of Adverse Reactions to PR Platelets