Hospital Medicine 5: Clinical
Session: Hospital Medicine 5: Clinical
526 - Efficacy of High-Flow Nasal Cannula and Maximum Flows at Which it Can Be Safely Used On An Inpatient Pediatric Ward
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 526.4651
Adam Whiteley, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Youmna Mousattat, West virginia university school of medicine , wvu medicine, Charleston, WV, United States; Mouna Chebib, Charleston Area Medical Center, Charleston, WV, United States

Adam Whiteley, MD (he/him/his)
Pediatric Critical Care Fellow
Children's Mercy Hospitals and Clinics
Kansas City, Missouri, United States
Presenting Author(s)
Background: High flow nasal cannula (HFNC) use in pediatric populations has become increasingly more common as a primary mode of respiratory support for conditions such as bronchiolitis and asthma exacerbations. As HFNC is a newer support device, there remains limited data on how much flow can be delivered safely in the inpatient setting outside of the pediatric intensive care unit (PICU)
Objective: 1) To determine if an increased level of HFNC support can be provided to patients at higher flow rates on the pediatric floor without increasing the frequency of emergent patient decompensation events and 2) To determine the impact of changing respiratory support guidelines on resource utilization
Design/Methods: This was an observational cohort study conducted via retrospective chart review. Patients admitted July 1, 2021 to December 31, 2023 meeting inclusion criteria were separated pre- (Cohort 1) and post- (Cohort 2) the change in our institutional HFNC flow guidelines (Fig 1). Inclusion and exclusion criteria can be seen in Table 1 and collected variables can be seen in Table 2. Descriptive analysis was conducted for each variable in this study with medians and interquartile ranges for continuous variables reported. For categorical variables, proportions and frequencies were computed. Statistical significance was determined with t tests or if needed Mann-Whitney U tests for continuous variables and chi-square or Fisher’s Exact test for categorical variables.
Results: 886 patients were initially identified with 734 patients ultimately included for analysis, 367 in each cohort. There were no statistically significant differences in cohort demographics: median age (yrs) 1.0 vs. 1.2 (p = 0.08), sex (M) 59.9% vs 58.0% (p=0.60), median weight (kg) 10.0 vs. 10.3 (p=0.20). Compared to cohort 1, cohort 2 had significant decreases in direct PICU admissions (4.4% vs 14.4%, p< 0.001), PICU transfers (4.6% vs. 14.6%, p< 0.001), length of stay (days) 2.0 vs. 3.0 (p < 0.001), and median cost $12555 vs. $14476 (p < 0.001). Cohort 2 had a less but not statistically significant different number of medical emergency team calls, 1 vs 3 (p=0.37). In cohort 1, 80 of 99 PICU admissions/transfers and a total of 240.5 PICU care days could have been prevented with the new HFNC guidelines. In cohort 2, 109 of 351 patients remained on the floor on HFNC rates greater than the old HFNC guidelines and a total of 170.5 PICU care days were prevented.
Conclusion(s): Higher rates of HFNC flow can be safely delivered on pediatric floors without an increase in adverse events while decreasing costs and preserving access to critical care services
Figure 1 - HFNC Flow Guidelines Before and After October 2022
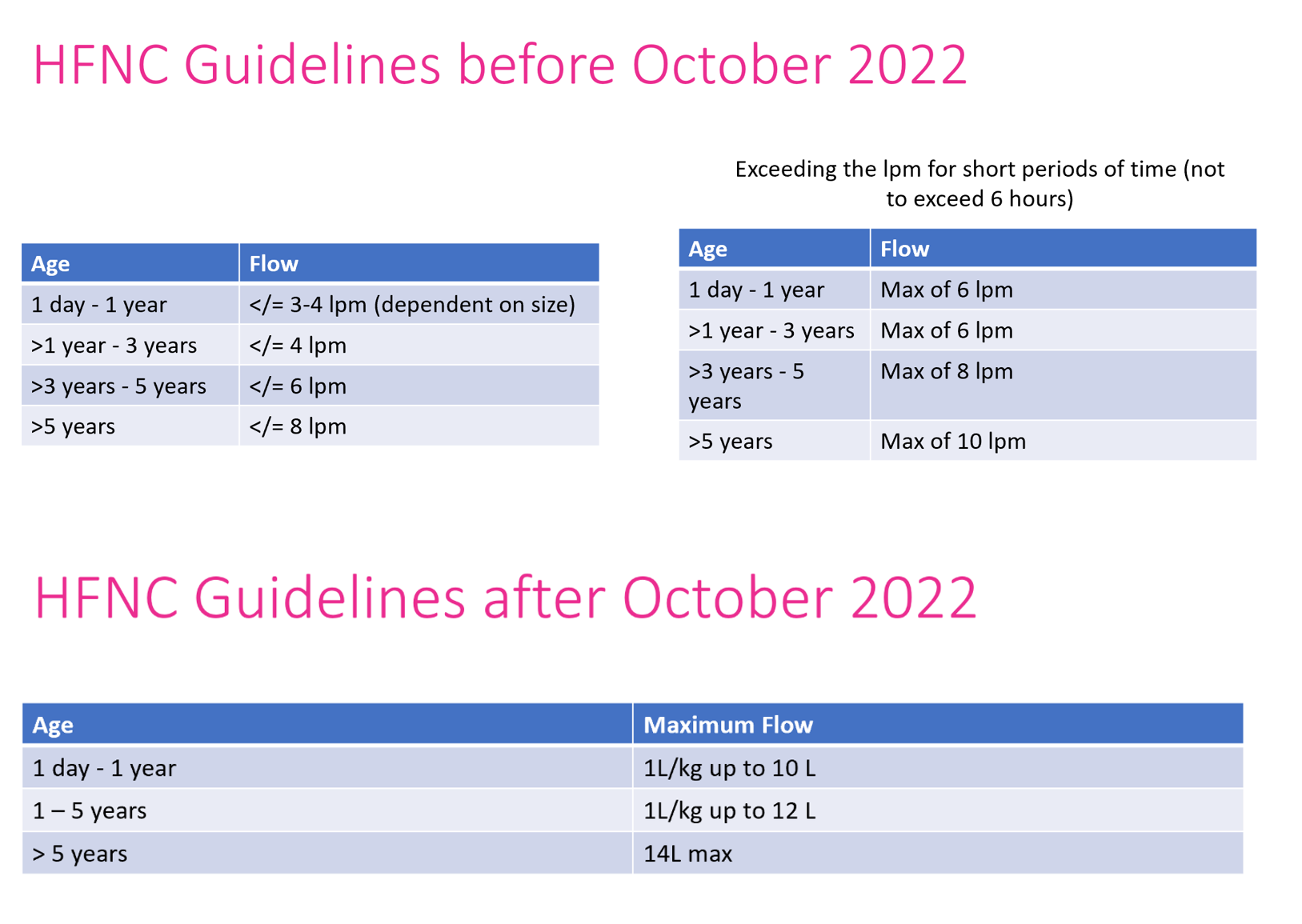
Table 1 - Study Criteria
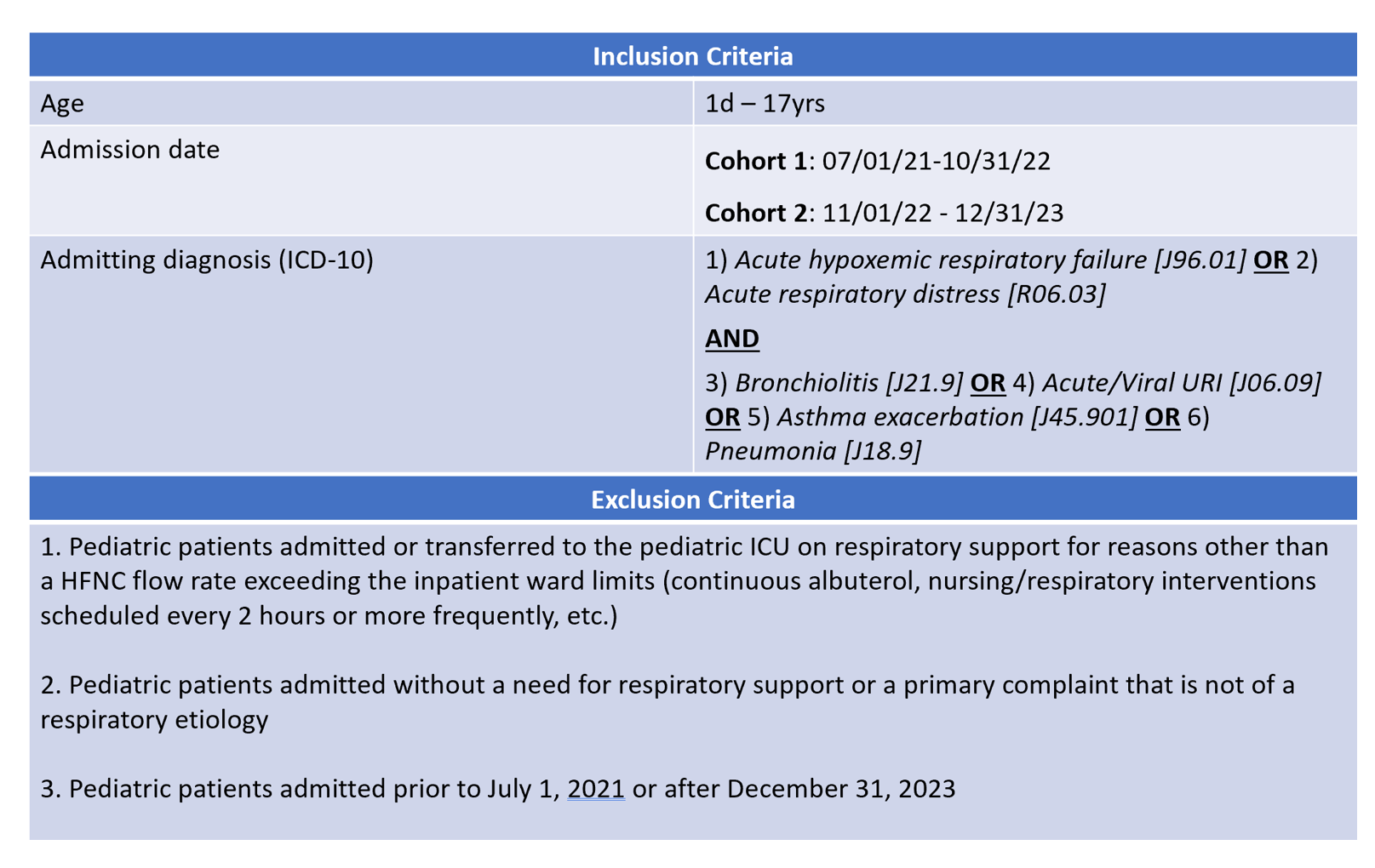
Table 2 - Study Variables
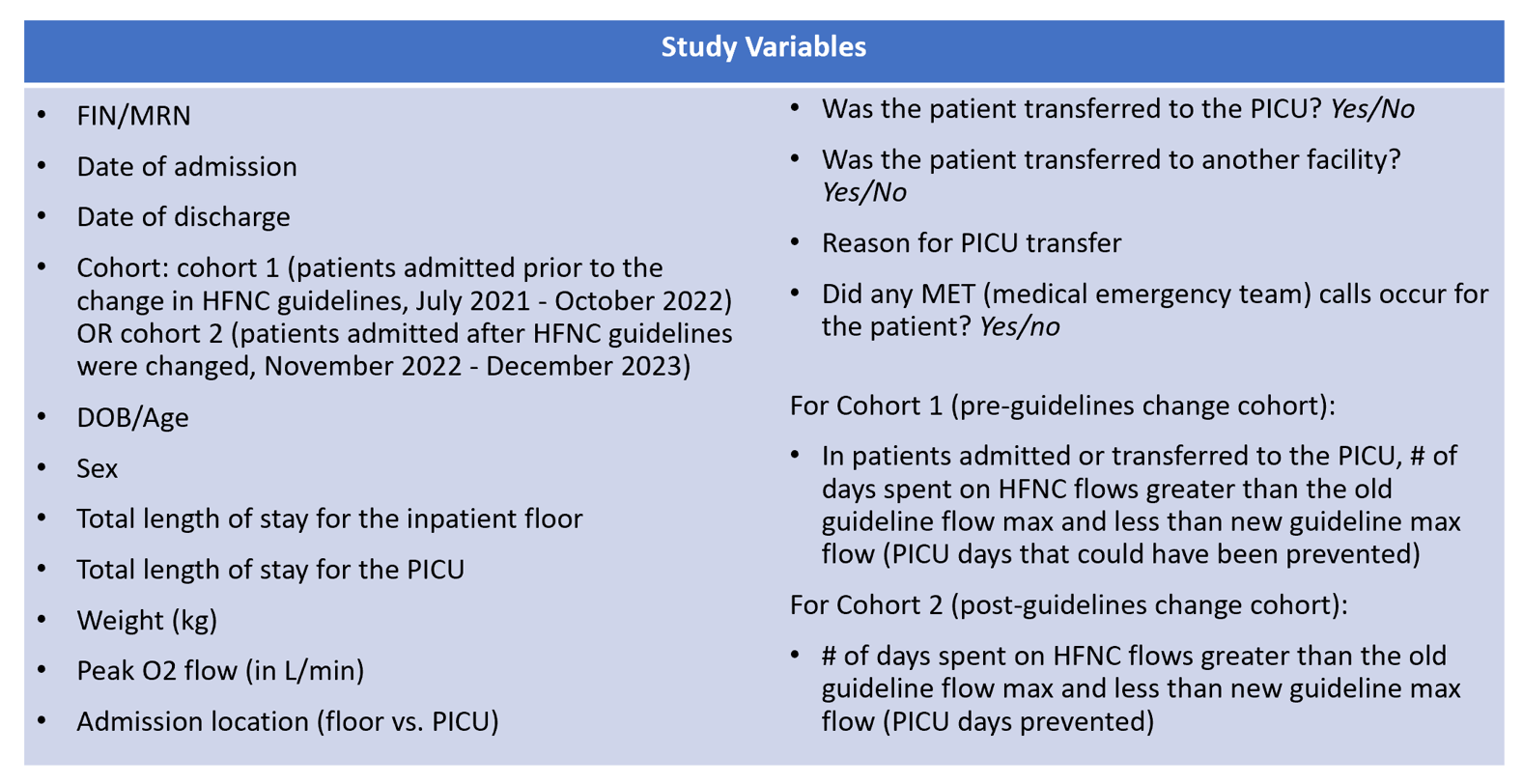
Figure 1 - HFNC Flow Guidelines Before and After October 2022

Table 1 - Study Criteria

Table 2 - Study Variables


