Neonatal Neurology 5: Fetal
Session: Neonatal Neurology 5: Fetal
661 - Sleep-Disordered Breathing Among Newborns With Fetal vs. Postnatal Myelomeningocele Closure
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 661.6624
John D. E.. Barks, University of Michigan Medical School, Ann Arbor, MI, United States; Ronald D. Chervin, University of Michigan Medical School, Ann Arbor, MI, United States; Jagruti Anadkat, Washington University in St. Louis School of Medicine, St. Louis, MO, United States; Ellen Bendel-Stenzel, Mayo Clinic Children's Center, Rochester, MN, United States; Stephanie Eyerly-Webb, Children's Hospitals and Clinics of Minnesota, Minneapolis, MN, United States; Fauziya Hassan, University of Michigan Medical School, Ann Arbor, MI, United States; Niko Kaciroti, University of Michigan Medical School, Ann Arbor, MI, United States; Robin Lloyd, Mayo Clinic Children's Center, Rochester, MN, United States; Thornton Mason, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States; Harlan McCaffery, University of Michigan Medical School, Ann Arbor, MI, United States; Lora Merley, University of Michigan Medical School, Holt, MI, United States; Ramesha Papanna, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; Stephanie Rau, University of Michigan Medical School, Ann Arbor, MI, United States; Regina M. Reynolds, Children's Hospital Colorado, Denver, CO, United States; Stefanie Riddle, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Brandon Rocque, University of Alabama School of Medicine, Birmingham, AL, United States; Renee A. Shellhaas, Washington University in St. Louis School of Medicine, St Louis, MO, United States
.jpg)
John D. E Barks, MD (he/him/his)
Professor
University of Michigan Medical School
Ann Arbor, Michigan, United States
Presenting Author(s)
Background: Sleep-disordered breathing (SDB) is common among children with myelomeningocele (MMC) and is a risk factor for sudden death in young adults with MMC. Yet, SDB screening is not routine, even in centers with specialized MMC programs.
Fetal surgery, compared to postnatal MMC repair, improves motor development and can reduce the need for ventriculoperitoneal (VP) shunts. However, the cognitive impairments associated with MMC are not improved by fetal surgery. For otherwise healthy children even mild symptoms of SDB during infancy identify long-term risk for subtle cognitive deficits. Thus, SDB may be a remediable contributor to abnormal cognitive outcomes for children with MMC.
Objective: We aimed to determine whether fetal vs. postnatal MMC repair influences neonatal SDB.
Design/Methods: In this 9-center, prospective study, decisions regarding fetal vs. postnatal surgery and treatment of SDB were made by the clinical teams. Neonates underwent a 10-hour bedside polysomnogram (PSG) at ≥35 weeks postmenstrual age (PMA), before hospital discharge. Imaging and PSGs were scored centrally by blinded experts. Multiple linear regression, adjusted for propensity to select fetal vs. postnatal surgery, was used to model predictors of SDB severity, as reflected by the apnea-hypopnea index (AHI; log-transformed due to skewed data). The study was powered with an a priori non-inferiority AHI margin of 10 events per hour, with a pre-specified sample size of n=173.
Results: Among 173 infants, 90 had fetal surgery and 83 had postnatal MMC repair, and 92/173 (53%) had SDB (Figures 1 & 2). In univariate analysis, AHI was higher among neonates with fetal rather than postnatal surgery (median 29, IQR 14-49, vs. 19, IQR 10-36, p=0.008). After adjusting for propensity for fetal surgery and PMA at the time of the PSG, there was no difference in AHI. Neither the need for VP shunt, nor hindbrain herniation, nor MMC level predicted AHI in adjusted models. Supplemental oxygen was the most common neonatal SDB treatment (prescribed for 43% of infants with fetal and 38% postnatal MMC repair, p=0.6).
Conclusion(s): In this well-powered non-inferiority study, we demonstrate that most neonates with MMC have SDB. The higher AHI in the fetal surgery group could be a function of a higher incidence of prematurity. SDB assessment may be appropriate as early as the first weeks after delivery for neonates with MMC. As SDB is treatable, early diagnosis and intervention could become an integral part of a multidisciplinary treatment strategy to optimize long-term medical and neurodevelopmental outcomes.
Table
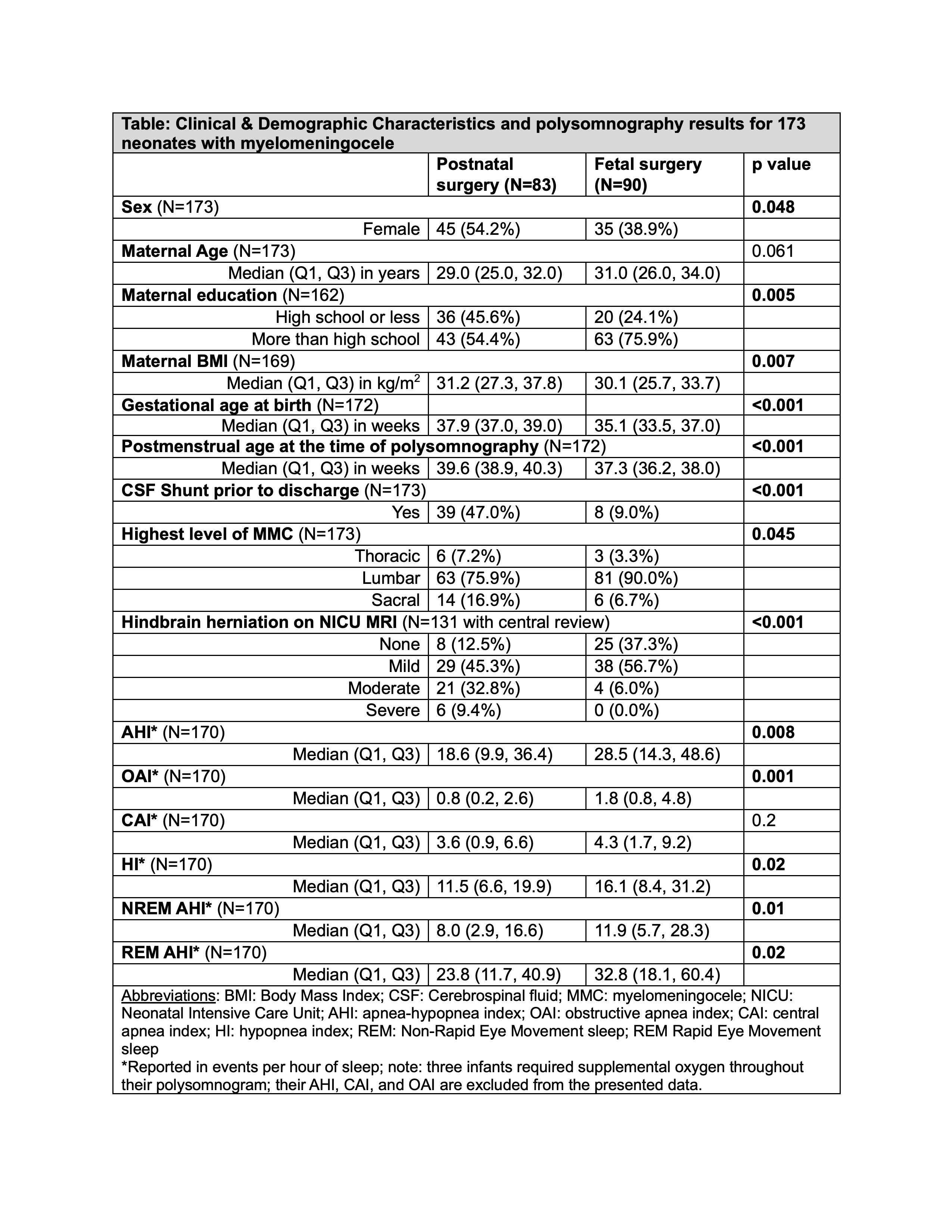 Clinical and demographic characteristics and polysomnography results for 173 neonates with myelomeningocele
Clinical and demographic characteristics and polysomnography results for 173 neonates with myelomeningoceleFigure 1
Figure 2
Table
 Clinical and demographic characteristics and polysomnography results for 173 neonates with myelomeningocele
Clinical and demographic characteristics and polysomnography results for 173 neonates with myelomeningoceleFigure 1
Figure 2

