Nephrology 1
Session: Nephrology 1
001 - Biopsy Findings from the VIRTUUS Study: A North American Cohort of Incident Pediatric Kidney Transplant Recipients
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 1.3915
SANDRA AMARAL, CHOP, Wynnewood, PA, United States; Tom Blydt-Hansen, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Asha Moudgil, Children's National Hospital, Silver Spring, MD, United States; Elizabeth Ingulli, UCSD, San Diego, CA, United States; Paul Grimm, Stanford University, Medical S, Palo Alto, CA, United States; Priya S. Verghese, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; David K. Hooper, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Jodi Smith, University of Washington School of Medicine, Seattle, WA, United States; Debora Matossian, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States; Sarah Kizilbash, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States; Annie Chung, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Rachel M. Lestz, Children's Hospital Los Angeles, Los Angeles, CA, United States; Jon Savant, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Brendan Keating, new york university langone health, New York, NY, United States; Juhi Kumar, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States

Sandra Amaral, MD, MHS (she/her/hers)
Assoc Prof
CHOP
PHILADELPHIA, Pennsylvania, United States
Presenting Author(s)
Background: The epidemiology of allograft histology in children in the first year post-kidney transplant is poorly described and transplant center biopsy practices vary widely. The VIRTUUS study is an observational cohort of pediatric academic transplant centers in the US and Canada working together to examine longitudinal associations of urine biomarkers with transplant histology and outcomes.
Objective: We sought to compare biopsy findings between incident pediatric kidney transplant recipients at VIRTUUS sites that perform surveillance biopsies vs. those doing for-cause biopsies only.
Design/Methods: Ten VIRTUUS sites recruited incident kidney transplant recipients < 19 years old and reported clinical and biopsy data across the first year post-transplant. Sites followed their standard biopsy practices. Biopsy data were adjudicated by two investigators using 2019 Banff criteria categories of normal, antibody-mediated changes, borderline and T-cell mediated rejection, interstitial fibrosis and tubular atrophy, and other. Descriptive statistics were used to compare histology between sites performing surveillance biopsies vs. for-cause biopsies only.
Results: 344 incident pediatric kidney transplant recipients underwent 399 kidney biopsies. Six sites performed surveillance biopsies (60%). Demographics of enrolled children are shown in Table 1. 97 children (28.2%) had one biopsy performed, 58 (16.9%) had two biopsies and 49 (14.2%) had more than 3 biopsies. 133 biopsies in 91children showed rejection. Table 2 shows differences in histology between sites performing surveillance biopsies vs. for-cause biopsies only. Sites performing surveillance biopsies had more biopsies that were normal (30.8%), and detected borderline rejection, calcineurin toxicity and pyelonephritis more commonly. Sites doing for-cause biopsies detected abnormalities in 83.5% of biopsies, most commonly t-cell mediated rejection, acute tubular injury and recurrent disease.
Conclusion(s): The VIRTUUS cohort represents a large, diverse cohort of children with incident kidney transplants cared for in sites with varying biopsy practices. The prevalence and variety of abnormal histology differed between sites that performed surveillance biopsies vs. for-cause biopsies only. Many of the findings detected by surveillance, such as calcineurin inhibitor toxicity, borderline rejection and pyelonephritis are actionable. Further studies will examine transplant outcomes, such as persistence of rejection and egfr at one year post-transplant, to advance understanding of the clinical utility of surveillance biopsies.
Table 1. Demographics of incident pediatric kidney transplant recipients in the VIRTUUS cohort.
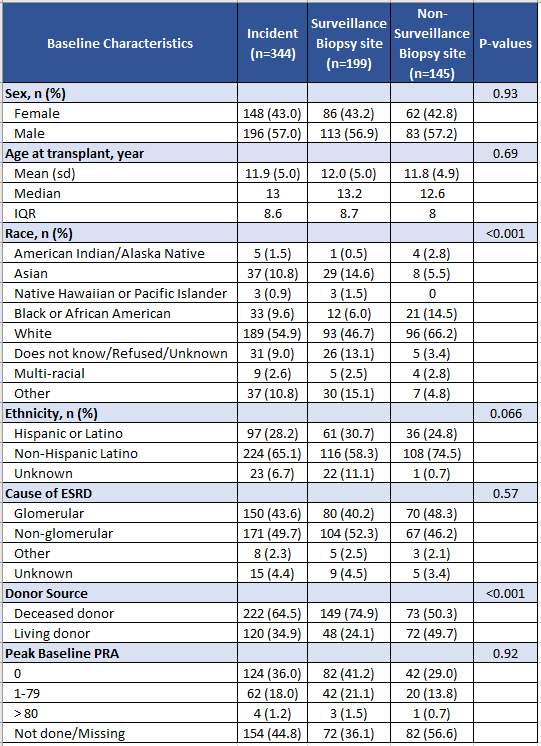
Table 2. Biopsy characteristics comparing sites that perform surveillance biopsies vs. those that do not.
.png)

