Newborn Care 1
Session: Newborn Care 1
242 - Congenital Cytomegalovirus Detection Following Pennsylvania Hearing-Targeted Screening Legislation
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 242.4123
Sarah A. Coggins, University of Pennsylvania/Children's Hospital of Philadelphia, Philadelphia, PA, United States; Samuel J. Garber, Perelman School of Medicine at the University of Pennsylvania, Philadephia, PA, United States; Karen M. Puopolo, Children's Hospital of Philadelphia, Philadelphia, PA, United States

Sarah A. Coggins, MD MSCE
Instructor of Pediatrics
University of Pennsylvania/Children's Hospital of Philadelphia
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: Cytomegalovirus causes congenital infection (cCMV) in approximately 1/200-1/250 US newborns each year. Infants with cCMV may have clinical signs of infection at birth, but may also appear well at birth yet develop progressive sensorineural hearing loss. Multiple US states now mandate CMV testing for newborns who fail hearing screening at birth, but the yield of this approach is unclear. Since 2022, Pennsylvania law requires birth hospitals offer families CMV testing and education if their newborn fails hearing screening at birth, or cannot be tested (due to preterm birth or illness severity) before 21 days of age.
Objective: To review results of birth hospital cCMV screening after state-mandated hearing-targeted legislation.
Design/Methods: Retrospective cohort study of all live births occurring at 3 birth centers in the University of Pennsylvania health system, 1/1/2023-8/31/2024. We identified all infants who had CMV testing by urine or saliva PCR at ≤21 days of age. We collected demographic data and test results from the medical record. Small for gestational age (SGA) and microcephaly were defined as birth weight or head circumference < 10th percentile for gestation based on Fenton 2013 growth charts.
Results: Among 20,171 live births, 2,151 infants (11%) had CMV testing at ≤21 days of age. CMV-tested infants were of significantly lower gestational age and birth weight and more likely to be male, compared to untested newborns (Table 1). Among those tested, 713 (33%) were tested due to 2 failed hearing screens; 647 (29%) due to clinical signs (SGA or microcephaly); and 145 (7%) due to both failed hearing testing and clinical signs (Table 2). Among infants with ultimately normal hearing and growth parameters, 315 had failed an initial hearing screen and the remaining 331 were preterm or critically ill infants unable to undergo hearing screening before 21 days. CMV was detected in 18 infants (0.8% of tested infants; 0.9 cases/1000 live births). Infants with cCMV were more likely to have SGA or microcephaly compared to CMV-negative infants (Table 3). Among infants with cCMV, the most common indications for screening were failed hearing screen (7/18) and SGA with microcephaly (5/18) (Table 2). Valganciclovir therapy was initiated among 6/18 infants (33%) before birth hospital discharge.
Conclusion(s): A combination of hearing-targeted and clinical sign-prompted CMV testing identified cCMV in approximately 1/1100 newborns over a 20-month period. One-third of those diagnosed were identified on the basis of failed hearing screening alone.
Table 1
.png)
Table 2
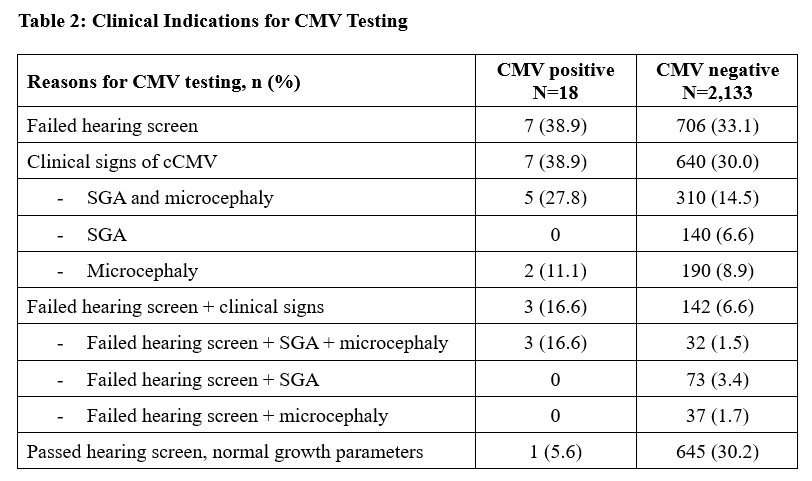
Table 3
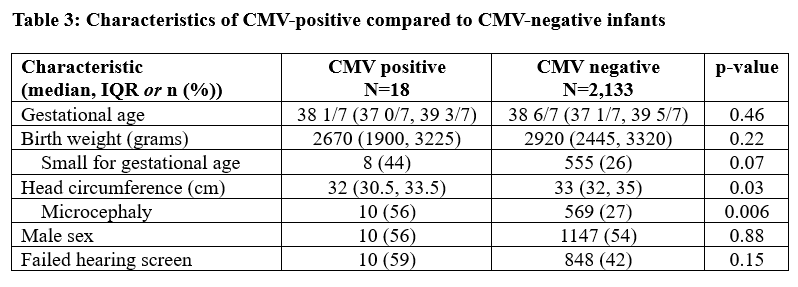
Table 1
.png)
Table 2

Table 3


