Asthma 1
Session: Asthma 1
466 - Comparison of High Flow Nasal Cannula to Bilevel Positive Airway Pressure for Pediatric Asthma Exacerbations
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 466.4070
Shelby Nelipovich, Golisano Children's Hospital at The University of Rochester Medical Center, Rochester, NY, United States; Lauren A. Ozdowski, Golisano Children's Hospital at The University of Rochester Medical Center, Rochester, NY, United States; Michele Smith, University of Rochester School of Medicine and Dentistry, Rochester, NY, United States

Shelby Nelipovich, MD (she/her/hers)
Resident
Golisano Children's Hospital at The University of Rochester Medical Center
Rochester, New York, United States
Presenting Author(s)
Background: Bilevel positive airway pressure (BPAP) and high flow nasal cannula (HFNC) are respiratory support modalities used to deliver beta agonist and improve respiratory mechanics in acute asthma exacerbations.
Objective: We aim to assess differences in characteristics and outcomes for patients admitted to the Pediatric Intensive Care Unit (PICU) with an asthma exacerbation who received either standard therapy (ST) alone (nebulized continuous beta agonist via facemask and systemic corticosteroids), ST plus HFNC, or ST plus BPAP.
Design/Methods: This observational retrospective and prospective cohort study included patients aged 3-17 years admitted to the PICU for an asthma exacerbation between 9/01/2019-9/01/22 and 10/01/23-5/01/24. Retrospective patients were identified using Virtual Pediatric Systems (VPS, LLC) with ICD-10 codes that included “Asthma.” Prospective patients were screened daily for eligibility. Individual charts were reviewed and data was recorded. The primary outcome assessed the duration of continuous beta agonist for patients with ST, ST plus HFNC, or ST plus BPAP. Secondary outcomes included PICU and hospital length of stay (LOS), respiratory support escalation, hourly respiratory rate (RR), use of sedatives and adjunct asthma therapies, and
adverse events. Chi-Squared/Fisher’s exact tests and the Kruskal Wallis test were used for categorical outcomes and continuous variables, respectively.
Results: 129 patients were included (ST n=57, HFNC n= 17, BPAP n=55). Patients placed on HFNC tended to be younger (median=4.5y, IQR 3.8-5.1) in comparison to BPAP (median=8.2y, IQR 5.1-10.4) and ST (median=6.8y, IQR 4.9-10.1). Patients on BPAP had longer duration of continuous beta
agonist (median=24.5hr, IQR=15.4-45.6) compared with ST and HFNC (p=0.0008 and p=0.04, respectively). No significant differences were found in escalation of respiratory support and adverse events. Patients on HFNC were least likely to require adjunct asthma medications (p=0.005). Sedation was most common in the BPAP group (p=0.03). The BPAP group had significantly longer PICU LOS (median=43.4hr, IQR 22.0-62.1) compared to ST (p=0.001) and HFNC (p=0.03) and a longer hospital LOS relative to the ST group (p=0.005). Analysis of RR during the first 12 hours of admission revealed increased trends in median RR for the HFNC group with relatively stable or decreasing median RR in the BPAP and ST groups.
Conclusion(s): The use of BPAP during pediatric asthma exacerbations is associated with longer duration of continuous beta agonist use, increased sedation use, and longer PICU and hospital LOS compared with patients on ST and HFNC.
Table 1. Duration of albuterol, respiratory support, PICU stay, hospital stay
.png)
Table 2. Pediatric Intensive Care Unit hospital course characteristics
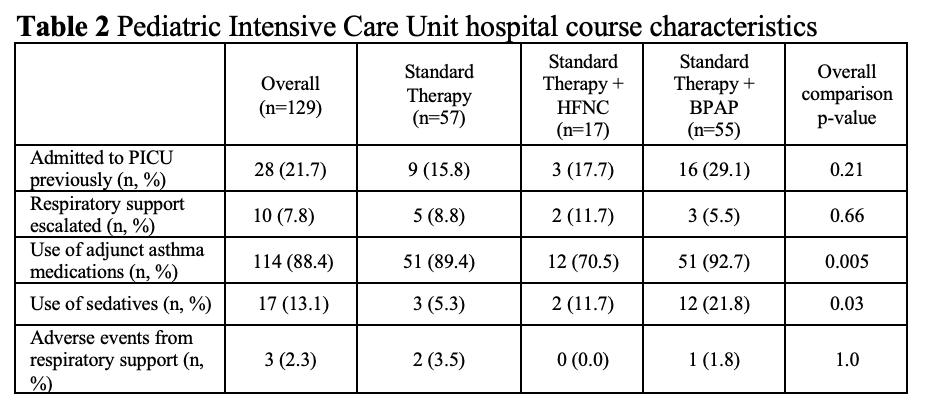
Figure 1. Respiratory rate over time for each respiratory support modality
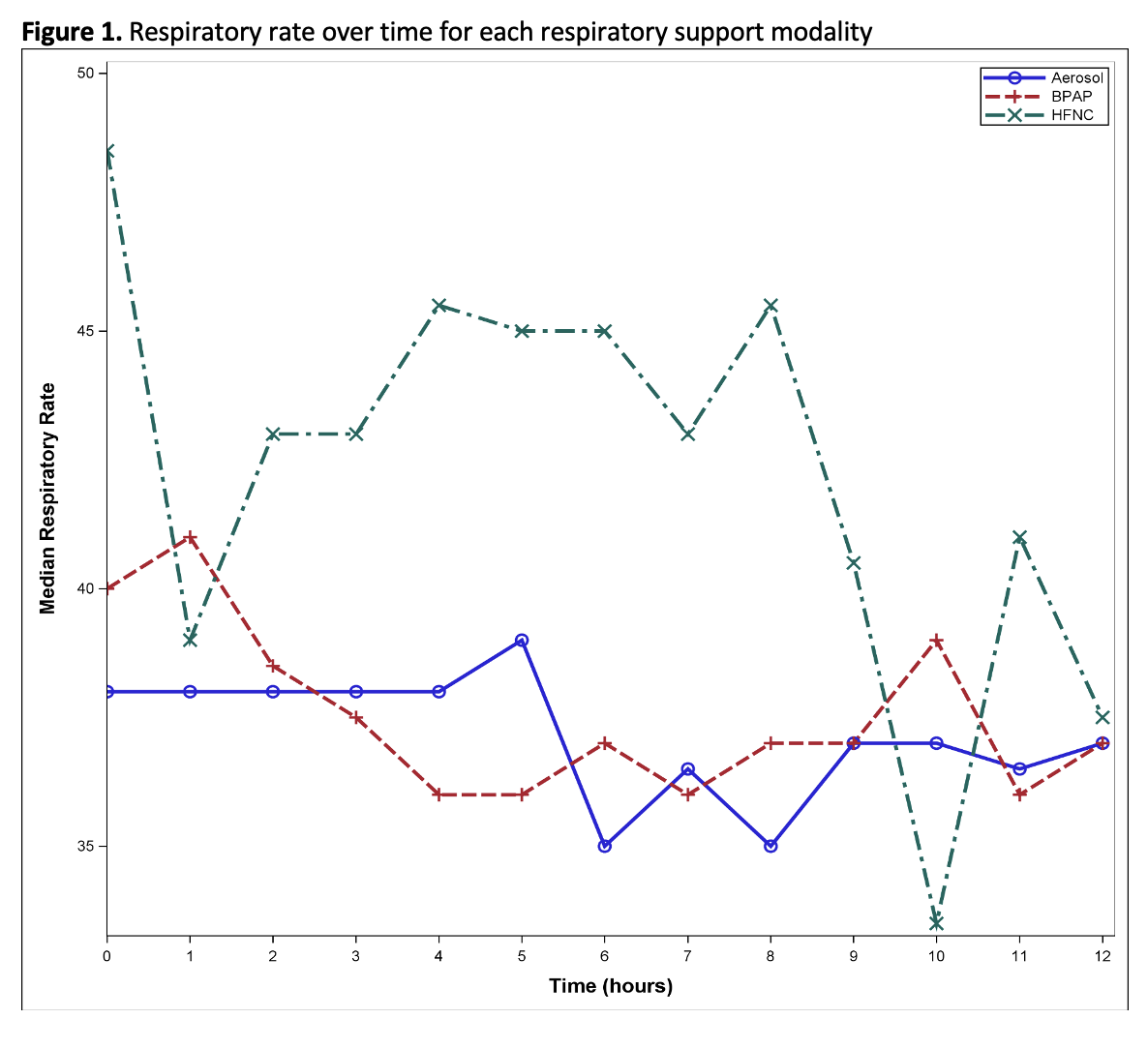
Table 1. Duration of albuterol, respiratory support, PICU stay, hospital stay
.png)
Table 2. Pediatric Intensive Care Unit hospital course characteristics

Figure 1. Respiratory rate over time for each respiratory support modality


