Neonatal Neurology 4
Session: Neonatal Neurology 4
646 - A Harmonized Database from Two Nationwide Hypoxic-Ischemic Encephalopathy Clinical Trials
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 646.3851
Chuan-Heng Hsiao, Boston Children's Hospital, Brookline, MA, United States; Anna N.. Foster, Boston Children's Hospital, Boston, MA, United States; Scott A.. McDonald, RTI International, Raleigh, NC, United States; Rutvi Vyas, Boston Children's Hospital, Boston, MA, United States; Aseelah Ashraf, Boston Children's Hospital, Harvard, Springfield, OH, United States; rina bao, Boston Children's Hospital, Boston, MA, United States; Lena Tran, University of Chicago, Boston, MA, United States; Ankush Kesri, Boston Children's Hospital, Boston, MA, United States; Erfan Darzidehkalani, Boston Childrens hospital, Boston, MA, United States; Matheus Dorigatti Soldatelli, Boston Children's Hospital, Boston, MA, United States; Jeanette O. Auman, RTI, Durham, NC, United States; Janet Soul, Boston Children's Hospital, Boston, MA, United States; Henry A.. Feldman, Boston Children's Hospital, Boston, MA, United States; Michael Cotten, Duke university, Durham, NC, United States; Seetha Shankaran, The University of Texas at Austin, Ann Arbor, MI, United States; Abbot Laptook, Women & Infants Hospital of Rhode Island, Providence, RI, United States; Patricia Ellen. Grant, Boston Children's Hospital, Boston, MA, United States; Yangming Ou, Boston Children's Hospital, Boston, MA, United States
- CH
Chuan-Heng Hsiao, MS (he/him/his)
Computational Biologist
Boston Children's Hospital
Brookline, Massachusetts, United States
Presenting Author(s)
Background: Hypoxic-ischemic encephalopathy (HIE) affects 1-5/1000 term neonates. Despite therapeutic hypothermia improving outcomes in high-income countries, 1/3 of patients with moderate to severe HIE still experience adverse neurocognitive outcomes by age 2 years. The Consortium Of Biomarkers In Neonatal Encephalopathy (COMBINE), established in 2022, aims to develop prognostic clinical and imaging biomarkers to predict adverse outcomes.
Objective: To develop a data harmonization framework, merge 2 trial datasets into a unified HIE database, and demonstrate suitability for prognostic biomarker development.
Design/Methods: Our first step is to merge comprehensive data from two nationwide therapeutic hypothermia for HIE trials (2008-2016, 21 sites) in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN). The 2 datasets differ in the available variables, variable naming and coding, necessitating harmonization.
We categorized the variables first by chronological stages (Pre-intervention, Intervention, Post-intervention, NICU-discharge, Follow Up, and Derived Data from the aforementioned 5 stages), and then by medical topics (maternal / infant characteristics, pregnancy, labor and delivery, birth condition, etc.). We created a dictionary to harmonize variable names and value coding. To demonstrate the utility of the merged database, we computed the distributions of variables and ranked them by the strength of associations with 18-22-month outcomes. Associations were measured by Pearson’s correlation or Spearman’s rank correlation. Outcomes were defined as (a) a 5-class variable: survivors with normal, mild, moderate, severe disability, or death; and (b) the Bayley-III scales in Cognition, Motor, and Language.
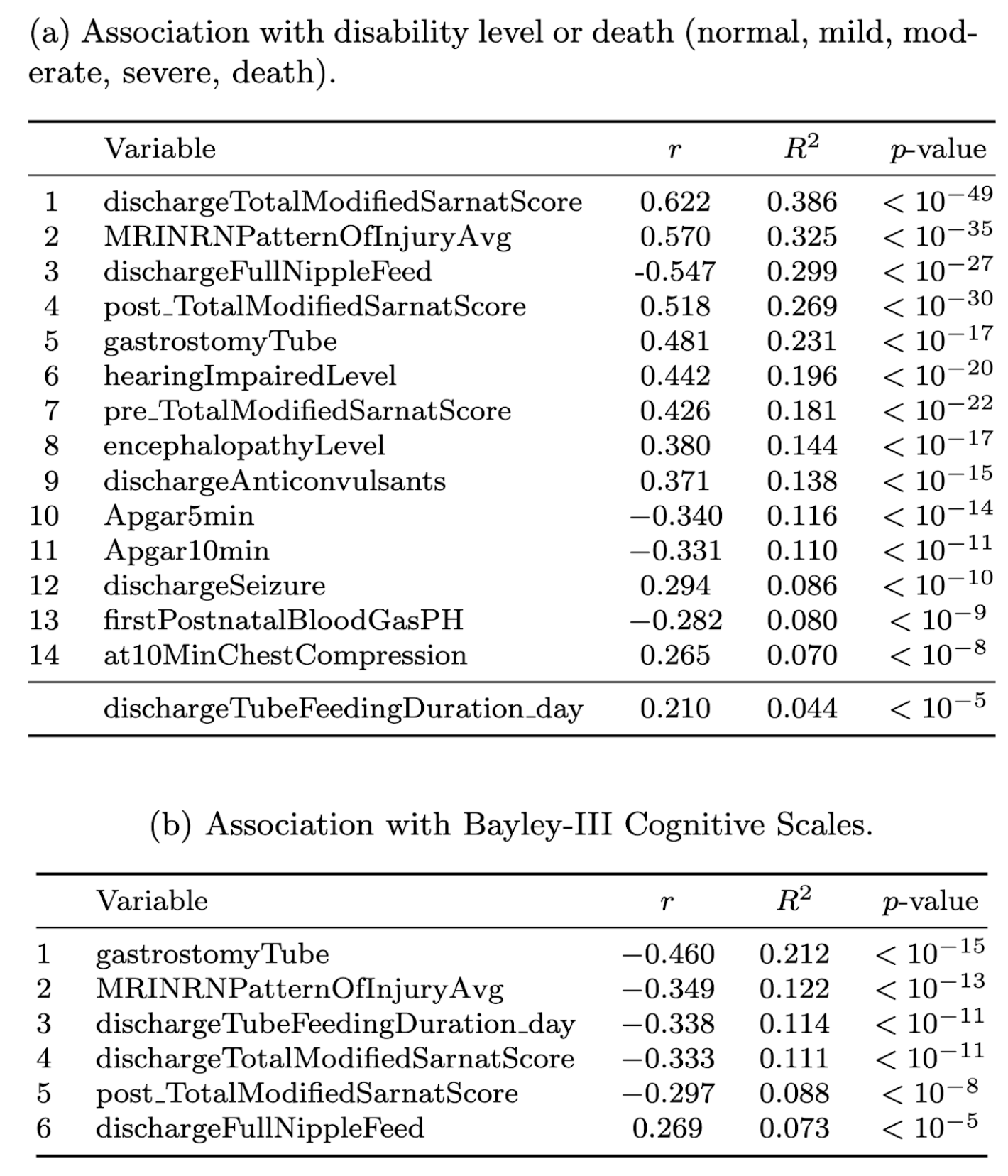
Results: We created a dictionary of 1345 variables (1079 from the Late Hypothermia trial, 2008-16, 21 sites; and 1204 from the Optimizing Cooling trial, 2010-14, 18 sites), on 532 patients across 6 chronologic categories and 62 medical sub-categories (Fig 1 for variable categories). Fig 2 illustrates the distribution of major predictive and outcome variables. Table 1 lists variables most strongly associated with neurocognitive outcomes at 18-22 months. The Sarnat exam at the NICU-discharge stage and the NRN pattern of MRI injury showed the strongest associations with disability level or death.
Conclusion(s): We designed a data harmonization framework specific for HIE. The merged database from 2 trials shows strong predictor-outcome associations, allowing subsequent development of multivariate prognostic biomarkers of neonatal HIE.
Categorization of the variables in the two HIE trials.

Distribution of 15 predictive variables and 5 outcome variables, from all 1345 variables in the merged database.
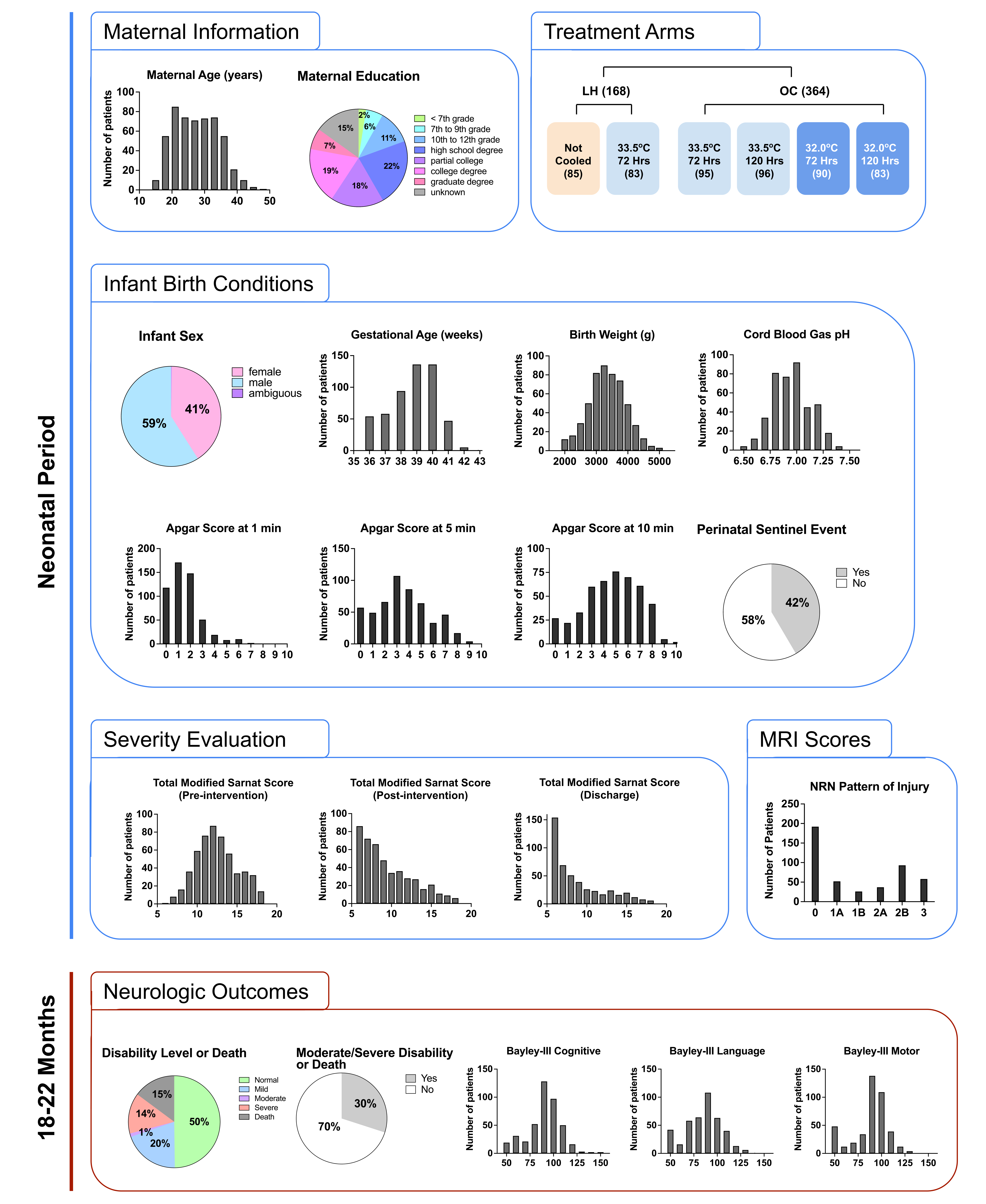
Variables with the strongest associations with outcomes at 18-22 months in the merged database.