Nephrology 1
Session: Nephrology 1
012 - Pediatric kidney allograft survival under tacrolimus-based immunosuppression with azathioprine compared to mycophenolate
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 12.6304
Eliza Blanchette, University of Colorado School of Medicine, Denver, CO, United States; Rodica Ciurea, Emmes, Rockville, MD, United States; Nicole Hayde, The Children's Hospital at Montefiore, White Plains, NY, United States; Mary Chandran, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, United States; Margret Bock, University of Colorado School of Medicine, Aurora, CO, United States; Tom Blydt-Hansen, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada

Eliza Blanchette, MD, MS (she/her/hers)
Assistant Professor
University of Colorado School of Medicine
Denver, Colorado, United States
Presenting Author(s)
Background: There is a lack of evidence in pediatric kidney transplant recipients (PKTR) on transplant outcomes amongst antiproliferative agents when combined with tacrolimus for maintenance immunosuppression.
Objective: To evaluate whether mycophenolate mofetil (MMF) remains superior to azathioprine (AZA) for allograft survival in the current era of tacrolimus-based immunosuppression (IS) in PKTR.
Design/Methods: This retrospective analysis of the NAPRTCS registry included PKTR from January 1, 2000, to December 31, 2019, on tacrolimus and either MMF or AZA at 1-month post-kidney transplant. Patients with kidney graft loss within 30 days were excluded. Participants were grouped as MMF only, AZA only or MMF to AZA switch after month-1 post-transplant. Allograft survival was modeled by participant group with adjustment for demographic and baseline characteristics, including the time to switch as a time dependent variable for those who switched from MMF to Aza.
Results: A total of 2,806 participants were included for analysis (MMF n=2462; AZA n=142; MMF to AZA = 202). The AZA, MMF to AZA and MMF differed respectively by age at transplant (mean age = 6.5 5.4 years, 9.0 5.7 years, 11.2 5.1 years; p < 0.0001), white race (64.1%, 64.9%, 53.2%; p=0.005), first transplants (71.8%, 61.4%, 74.0%; p=0.0018), deceased donor (44.4%, 42.1% vs. 52.2%; p=0.022), and had different induction therapy (p < 0.0001) and primary disease profiles (p < 0.0001), respectively (Table 1). Survival analysis identified differences in survival between the groups, p = 0.007 (Figure 1). The adjusted Cox PH model did not identify any survival advantage for MMF compared with Aza, using AZA as a time-dependent covariate, p = 0.65 (Table 2). The model findings were not affected by exclusion of patients treated with Alemtuzumab for induction immunosuppression.
Conclusion(s): These data do not identify a graft survival advantage for MMF, compared to patients with de novo AZA or MMF switch to AZA, while on tacrolimus-based immunosuppression. Future studies are needed to evaluate other kidney allograft outcomes.
Table 1. Study Population Baseline Characteristics Per Immunosuppression Therapy Groups
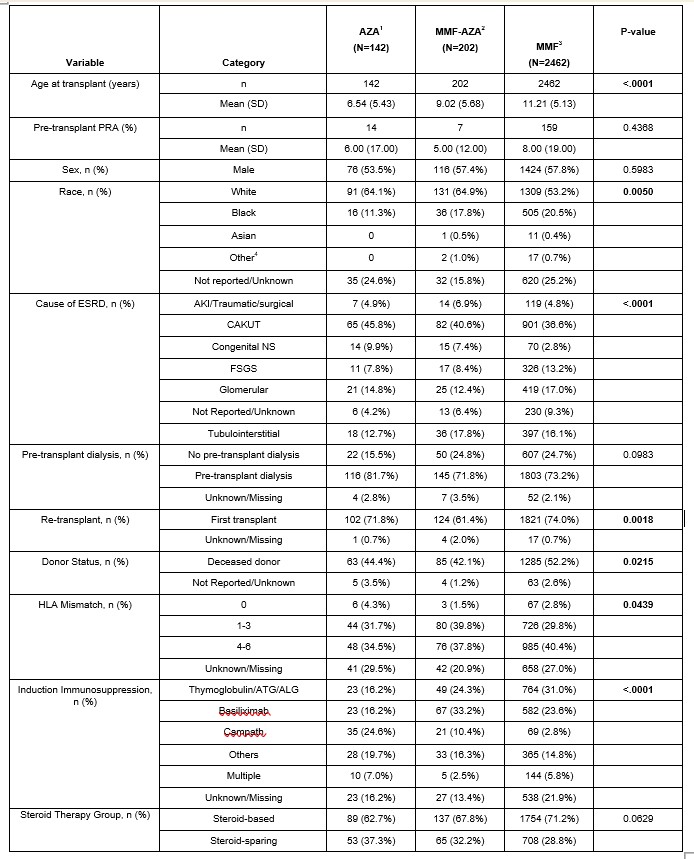 1. AZA group is defined as AZA use at the 30-day post-transplant visit.
1. AZA group is defined as AZA use at the 30-day post-transplant visit. 2. MMF-AZA group is defined as MMF use at 30-day post-transplant visit and subsequent switch to AZA.
3. MMF group is defined as MMF use at 30-day post-transplant visit and didn’t switch to AZA later.
4. American Indian or Alaskan Native, Native Hawaiian or Pacific Islander and Multi-racial were all combined into the “Other” race category.
Figure 1. Kaplan Meier for Graft Loos Stratified by Immunosuppression Groups
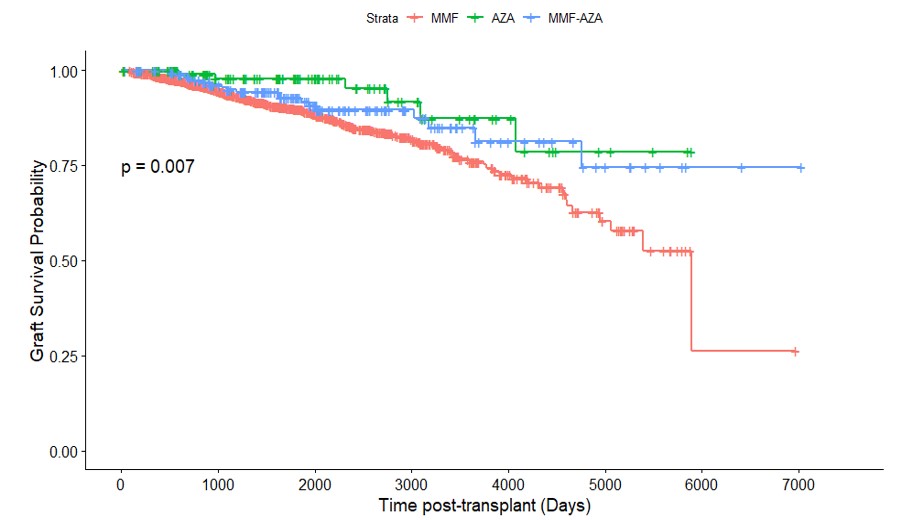
Table 2. Cox proportional hazards model results with first AZA use as time-dependent covariate.
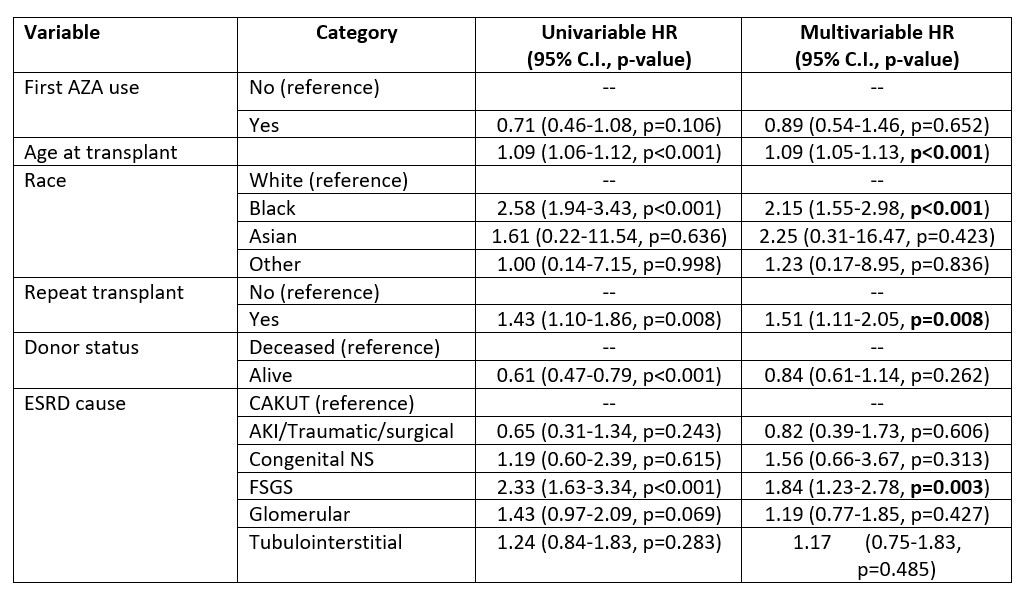 Alemtuzumab induction use is excluded
Alemtuzumab induction use is excluded
