Nephrology 1
Session: Nephrology 1
011 - Early Tacrolimus Variability and the Risk of Graft Loss in Pediatric Kidney Transplant Recipients
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 11.4818
Lidan Gu, University of Minnesota Medical School, Minneapolis, MN, United States; Finola E. Kane-Grade, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States; Michael Evans, University of Minnesota, Minneapolis, MN, United States; Danielle Glad, Medical College of Wisconsin, Lannon, WI, United States; Christopher J. Anzalone, Boston Children's Hospital, Waltham, MA, United States; Chelsey Jensen, M Health Fairview, Plymouth, MN, United States; Sarah Kizilbash, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States

Lidan Gu, Psychologist (she/her/hers)
Assistant Professor
University of Minnesota Medical School
Minneapolis, Minnesota, United States
Presenting Author(s)
Background: Nonadherence is a leading cause of graft loss in pediatric kidney transplant recipients, affecting approximately 30% of patients (Dobbels et al., 2010). The Medication Level Variability Index (MLVI), calculated as the standard deviation (SD) of serial blood drug levels, has been employed in liver, heart, kidney, and lung transplant recipients to assess nonadherence. These studies, however, mostly focus on patients who were at least one year post-transplant and suggest calculating MLVI based on serial drug levels over 1 year or longer time frame with a MLVI cut-off of 2 to indicate nonadherence (Shemesh et al., 2004; Eaton et al., 2018; Bilhartz et al., 2015; Duncan‐Park et al., 2022; Oliveira et al., 2017).
Objective: Our study aimed to determine whether MLVI within the first year and within shorter intervals (3- to 6-month) would predict graft loss in pediatric kidney recipients, and if the threshold of 2 is applicable to these early time frames.
Design/Methods: Our retrospective study included pediatric kidney transplant recipients (age < 21 years) transplanted at the University of Minnesota from 2010 to 2023 and who received tacrolimus for maintenance immunosuppression. MLVI, calculated as the SD of serial tacrolimus trough levels, was assessed across 4 posttransplant intervals with the following target trough levels: 2-week to 3-month (10–12 ng/mL); 3 to 6-month (8–10 ng/mL); 6 to12-month (6–8 ng/mL); and 12 to 24-month (4–6 ng/mL). Associations between MLVI and graft loss were analyzed using landmark survival analysis, with survival trees identifying optimal thresholds.
Results: The study included 160 pediatric recipients (mean age 12.5 years, IQR:5.8, 16.2), comprising 69% white, 10% Indigenous/Alaskan Native, 9% Asian American, 11% Black, and 1% Hawaiian/Pacific Islander. Median MLVI (IQR) values across the intervals are shown in Figure 1. Elevated MLVI during the 2-week to 3-month interval (HR: 1.68, 95% CI: 1.09–2.58, p=0.02) and 6 to 12-month interval (HR: 2.8, 95% CI: 1.26–2.63, p=0.01) was associated with increased risks of graft loss after 3 months and 12 months posttransplant, respectively. No significant associations were noted for the 3–6 or 12–24-month intervals. Survival tree analyses identified thresholds of 4.3 for the 2-week to 3-month and 2.4 for the 6 to 12-month intervals.
Conclusion(s): MLVI was significantly associated with graft loss in the 2-week to 3-month and 6 to 12-month intervals post-transplant. Monitoring MLVI during these periods may facilitate early identification of nonadherence, enabling timely interventions to reduce the risk of graft loss.
Figure 1 The median and interquartile range of the tacrolimus MLVI within the first 2 years of post-transplant
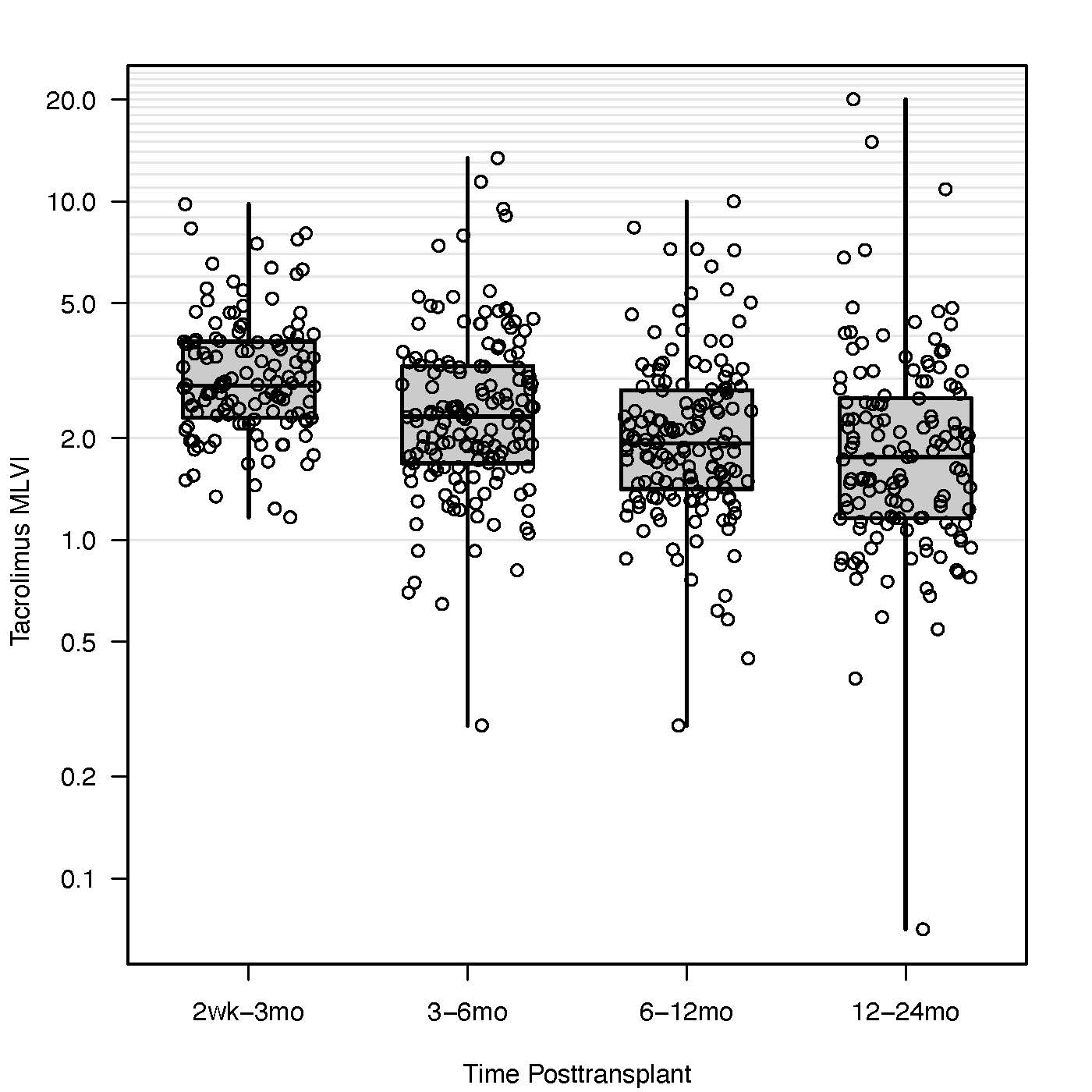
Table 1 Landmark survival analysis of graft loss
.jpg)

