Neonatal Neurology 4
Session: Neonatal Neurology 4
656 - Pharmacokinetics of RLS-0071, a Novel Anti-Inflammatory Peptide, in Newborns With Moderate or Severe Hypoxic Ischemic Encephalopathy
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 656.6106
Zachary A. Vesoulis, Washington University in St. Louis School of Medicine, St. Louis, MO, United States; Kenji Cunnion, Children's Hospital of The King's Daughters, Norfolk, VA, United States; Neel K. Krishna, ReAlta Life Sciences, Norfolk, VA, United States

Zachary A. Vesoulis, MD MSCI (he/him/his)
Assistant Professor
Washington University School of Medicine
St. Louis, Missouri, United States
Presenting Author(s)
Background: Hypoxic ischemic encephalopathy (HIE) is a common cause of mortality and life-long disabilities. RLS-0071 is a novel anti-inflammatory peptide that inhibits complement activation at C1, as well as myeloperoxidase activity and extracellular trap formation. PK analysis of RLS-0071 in animal and adult human studies demonstrate a two-compartment model with a bi-exponential decline in plasma concentration driven by rapid distribution into tissues. RLS-0071 is currently being evaluated in a phase 2 clinical trial of neonates with moderate or severe HIE.
Objective: Assess the PK profile of RLS-0071 in HIE babies for safety.
Design/Methods: STAR is a phase 2 randomized placebo-controlled dose-escalation trial (NCT05778188) at 13 NICUs in the USA. After informed consent, RLS-0071 was administered within 10 hours of birth at 3 mg/kg IV (first dosing cohort) and continued every 8 hours for a total of 10 doses. Blood samples were obtained pre-dose, end of first infusion (Cmax), 1 hour post dose (at the inflection point in the bi-exponential kinetics) and at 8 hours post dose (Cmin). Plasma samples were processed to isolate the peptide prior to measurement by LC/MS (Bioagilytix).
Neonatal PK modeling was performed using a 2-compartment model and results compared against adult PK profiles derived in a different clinical trial (NCT05298787). To simulate a pediatric population, an adult model was adapted with 30% variability in clearance (CL) and volume of distribution (Vd). This model was then applied to neonates weighing 3 kg, with 100 simulations performed by sampling from the ETA distributions of the PK parameters. Simulations were conducted using allometric scaling and assumed a conservative 50% reduction in CL to account for delayed organ maturation and hypothermia.
Results: RLS-0071 plasma concentrations were obtained for 10 HIE neonates (8 moderate, 2 severe) and summarized in Table 1. HIE infant PK curves for RLS-0071 at the 3 mg/kg/dose were similar to adult human curves at the 2 mg/kg/dose (Figure 1) and within established safe plasma levels in adults. No drug accumulation was observed. Simulation results predicted that 30% of infants receiving a dose of 3 mg/kg would reach the target peak concentration (Cmax = 17 mcg/ml), associated with optimal efficacy in the animal model (Table 2).
Conclusion(s): The kinetics of RLS-0071 were bi-exponential and fell within previously established safety limits. These results are reassuring for the continued clinical trials of RLS-0071 in HIE. Additional analysis is planned for higher dosing cohorts.
Table 1. PK plasma values for RLS-0071 at 3mg/kg IV q8hr
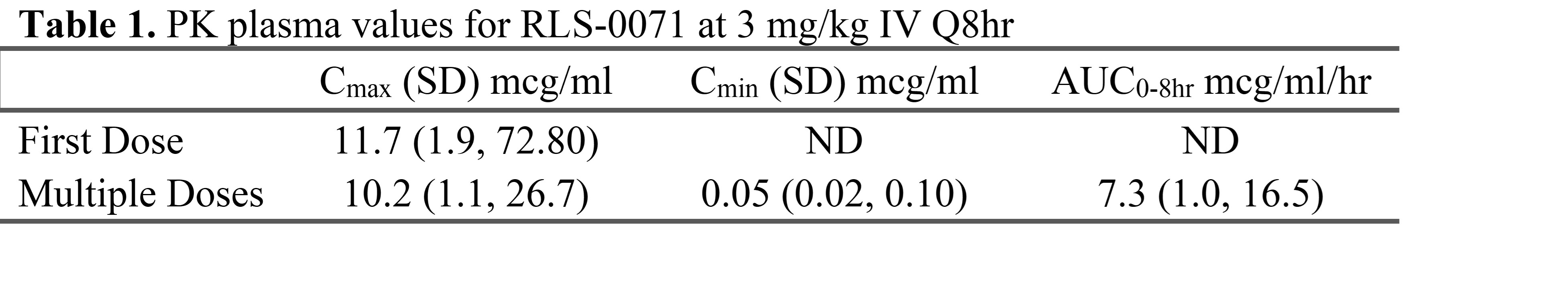
Table 2. Predicted proportion of participants about 17 mcg/ml threshold
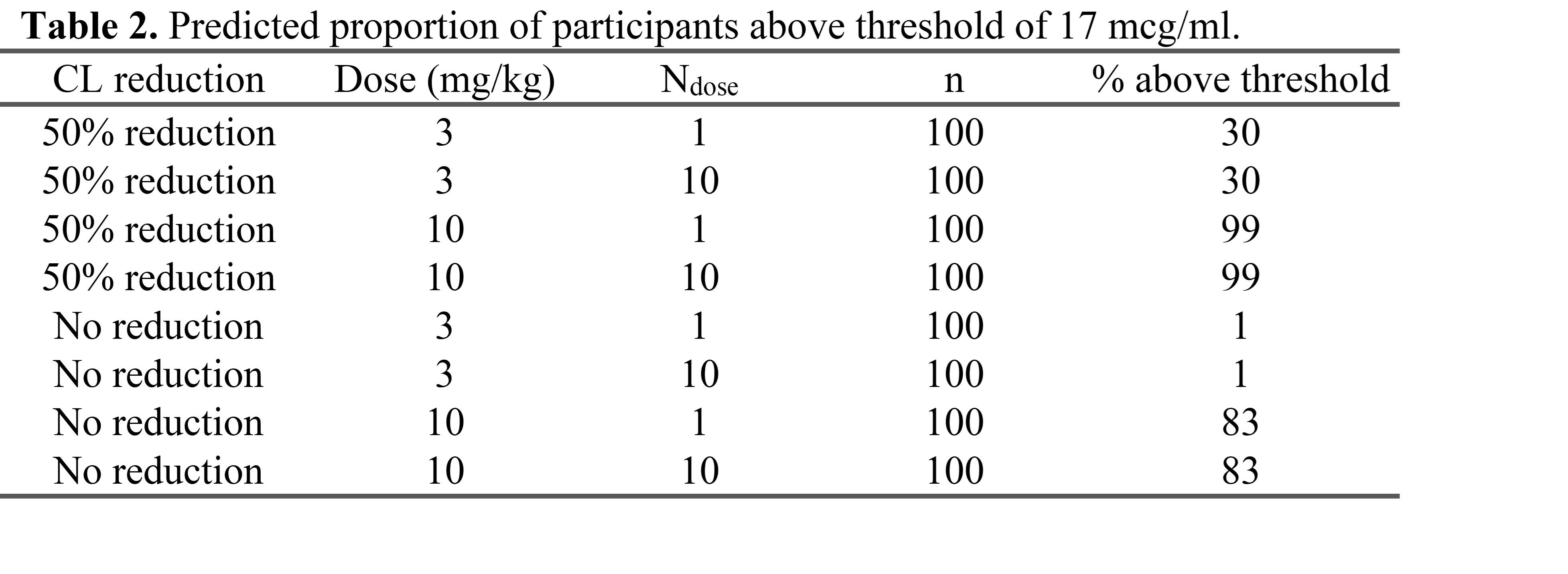
Figure 1. Comparison of pediatric and adult RLS-0071 PK curves
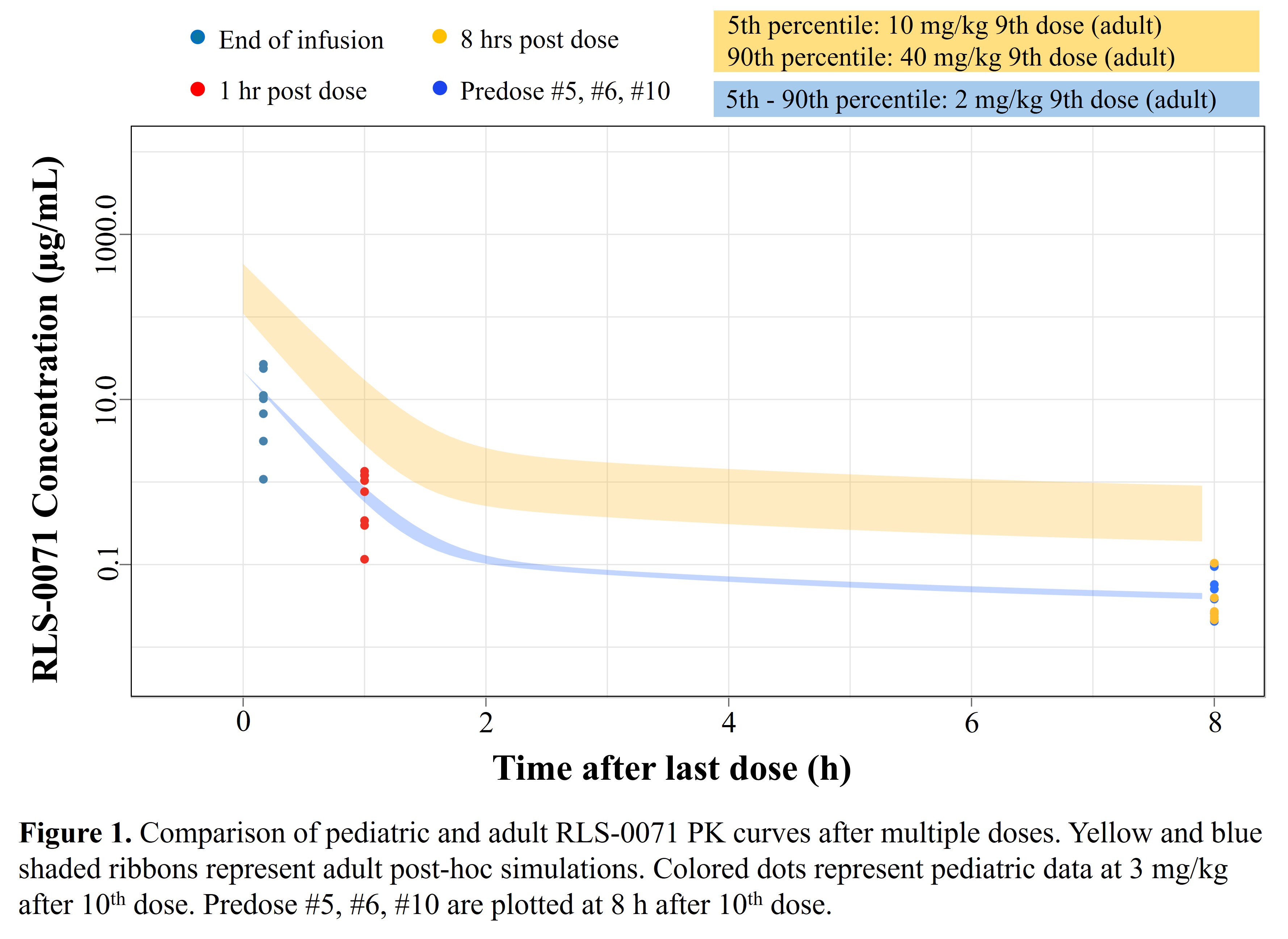 Yellow and blue shaded ribbons represent adult post-hoc simulations. Colored dots represent pediatric data at 3mg/kg after 10th dose. Predose #5, #6, #10 are plotted at 8 hours after 10th dose.
Yellow and blue shaded ribbons represent adult post-hoc simulations. Colored dots represent pediatric data at 3mg/kg after 10th dose. Predose #5, #6, #10 are plotted at 8 hours after 10th dose.Table 1. PK plasma values for RLS-0071 at 3mg/kg IV q8hr

Table 2. Predicted proportion of participants about 17 mcg/ml threshold

Figure 1. Comparison of pediatric and adult RLS-0071 PK curves
 Yellow and blue shaded ribbons represent adult post-hoc simulations. Colored dots represent pediatric data at 3mg/kg after 10th dose. Predose #5, #6, #10 are plotted at 8 hours after 10th dose.
Yellow and blue shaded ribbons represent adult post-hoc simulations. Colored dots represent pediatric data at 3mg/kg after 10th dose. Predose #5, #6, #10 are plotted at 8 hours after 10th dose.
