Nephrology 1
Session: Nephrology 1
014 - Biometric and biochemical assessment of vascular stiffness in pediatric kidney transplant recipients
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 14.3642
Yitong A. Liu, University of New Mexico School of Medicine, Albuquerque, NM, United States; Brandy B. Markley, Washington University, Wildwood, MO, United States; Raja Dandamudi, Washington University in St. Louis School of Medicine, St.Louis, MO, United States; Vikas Dharnidharka, Rutgers, Robert Wood Johnson Medical School, New Brunswick, NJ, United States; Keith Hruska, Washington University in St. Louis School of Medicine, St Louis, MO, United States

Yitong A. Liu, MD (he/him/his)
Assistant Professor
University of New Mexico School of Medicine
Albuquerque, New Mexico, United States
Presenting Author(s)
Background: Chronic kidney disease mineral bone disorder (CKD-MBD) is a complex disease characterized by disruptions in bone physiology, phosphorous and calcium homeostasis, and cardiovascular dysfunction. Among the chronic complications of CKD-MBD is vascular calcification, which is a mediator of chronic cardiovascular morbidity and mortality in patients with CKD. Vascular calcification occurs through vascular smooth muscle (VSMC) osteochondrogenic transdifferentiation.
Changes of transdifferentiation are noted as early as stage 2 CKD, and continues to progress with CKD. The changes in vascular stiffness have not been shown to improve after initiation of dialysis, with scarce and inconsistent data on changes after kidney transplant. Further assessment of vascular stiffness using non-invasive techniques such as pulse wave analysis (PWA) in the post-transplant setting is therefore of interest.
Objective: To characterize VSMC behavior in the first year post-transplant by assessing its overall vascular phenotype and biochemical signaling using biometric measurements of vascular stiffness and laboratory evaluation of various protein signals.
Design/Methods: Retrospective PWA data was obtained on transplant patients through the first year post-transplant. These data were analyzed for trends in vascular stiffness and compared to biochemical studies performed on serum samples obtained at early (1-2 months post-transplant) and late (10-11 months post-transplant) parts of the first year post-transplant. ELISA was used to assess sclerostin (SOST) and osteoprotegerin (OPG) to assess the biochemical changes.
Results: 25 kidney transplant patients were included based on availability of PWA data. There was wide variability in PWA results, but no significant change over the first year. Biochemical assessment noted increased SOST from the early (month 1 and 2) to the late (month 10 and 11) of the first post-transplant year. OPG decreased slightly though the same period, though not in a statistically significant manner.
Conclusion(s): In our data, vascular stiffness is stable but does not improve over the first post-transplant year. Concurrently, SOST levels rise with concurrent OPG levels decrease that is not statistically significant. These have been reported in previous adult studies. The SOST rise may be resultant of multiple factors including transplant immunosuppression, change in activity, as well as ongoing or restored pediatric growth after kidney replacement by transplant.
Figure 1. Loess plot of AIx over time after kidney transplant
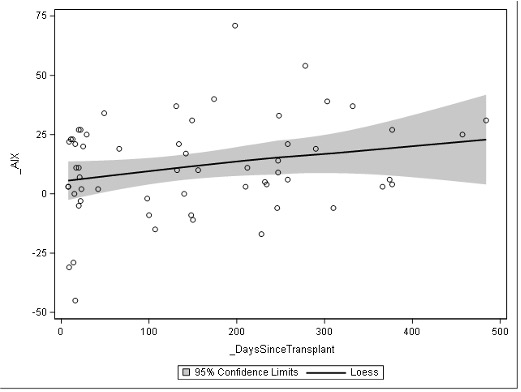 Loess plot of individual actuation index levels over time in days
Loess plot of individual actuation index levels over time in daysFigure 2. Comparison of AIx and central SBP in patient with and without dialysis history
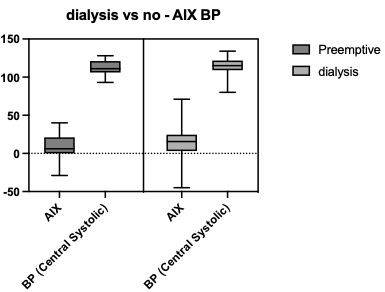 No significant difference was noted in actuation index or central systolic blood pressure ranges in kidney transplant patients who did and did not receive dialysis (either HD or PD) prior to kidney transplant
No significant difference was noted in actuation index or central systolic blood pressure ranges in kidney transplant patients who did and did not receive dialysis (either HD or PD) prior to kidney transplant Figure 3. Sclerotin trend after kidney transplant
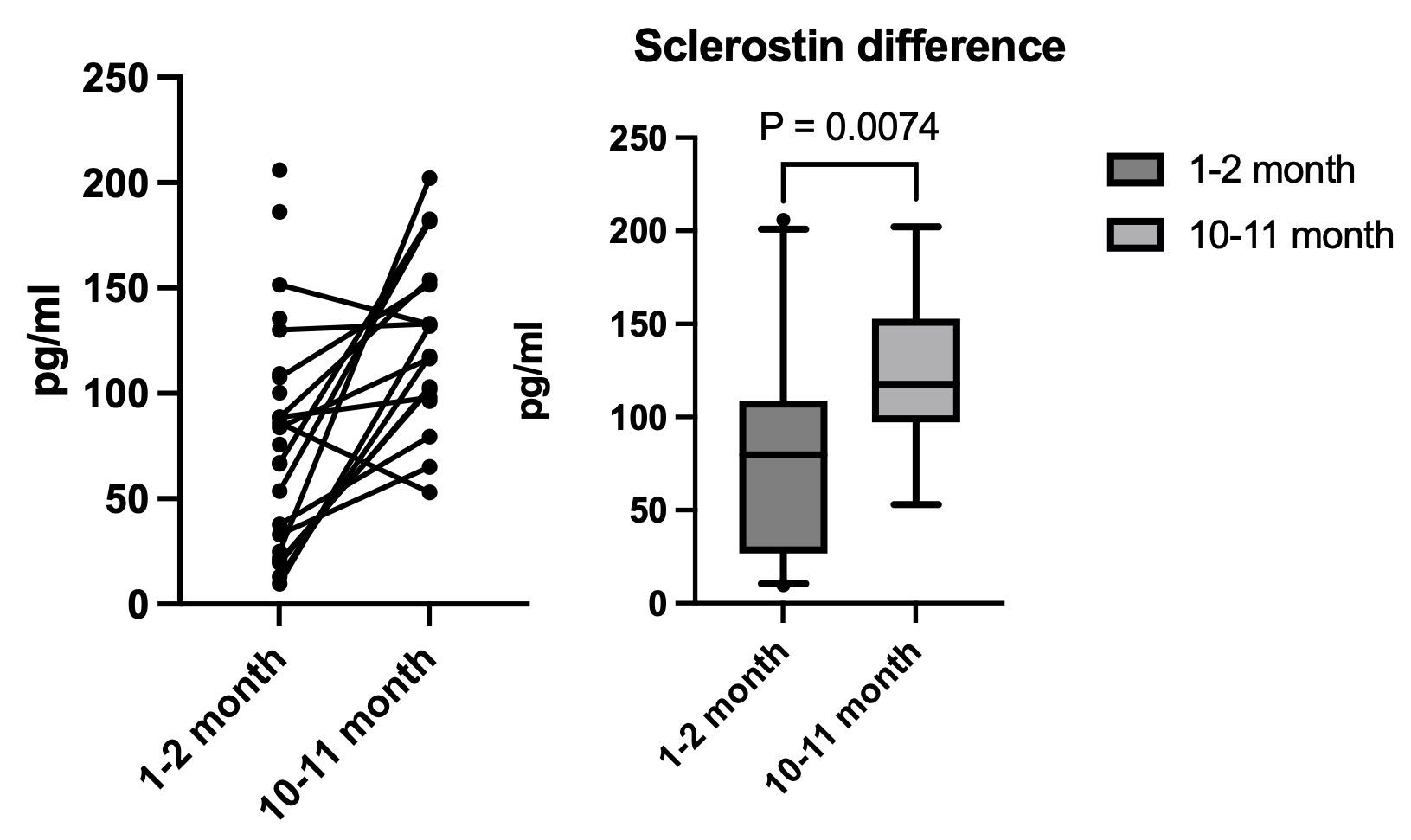 Sclerotin levels via ELISA in early (1-2 months) and late (10-11 months) in the first post-transplant year. There is a modest rise in the sclerotin level over the first transplant year (p <0.01).
Sclerotin levels via ELISA in early (1-2 months) and late (10-11 months) in the first post-transplant year. There is a modest rise in the sclerotin level over the first transplant year (p <0.01).Figure 1. Loess plot of AIx over time after kidney transplant
 Loess plot of individual actuation index levels over time in days
Loess plot of individual actuation index levels over time in daysFigure 2. Comparison of AIx and central SBP in patient with and without dialysis history
 No significant difference was noted in actuation index or central systolic blood pressure ranges in kidney transplant patients who did and did not receive dialysis (either HD or PD) prior to kidney transplant
No significant difference was noted in actuation index or central systolic blood pressure ranges in kidney transplant patients who did and did not receive dialysis (either HD or PD) prior to kidney transplant Figure 3. Sclerotin trend after kidney transplant
 Sclerotin levels via ELISA in early (1-2 months) and late (10-11 months) in the first post-transplant year. There is a modest rise in the sclerotin level over the first transplant year (p <0.01).
Sclerotin levels via ELISA in early (1-2 months) and late (10-11 months) in the first post-transplant year. There is a modest rise in the sclerotin level over the first transplant year (p <0.01).
