Nephrology 3
Session: Nephrology 3
588 - ENPP6 is a novel candidate gene for congenital anomalies of the kidneys and urinary Tract
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 588.4768
Nils D. Mertens, Boston Children's Hospital, Cambridge, MA, United States; Gijs Franken, Boston Children's Hospital, Boston, MA, United States; Shirlee Shril, Boston Children's Hospital, Boston, MA, United States; Junken Aoki, University of Tokyo, Tokyo, Tokyo, Japan; Friedhelm Hildebrandt, Boston Children's Hospital, Boston, MA, United States

Nils D. Mertens, MD (he/him/his)
Resident
Boston Children's Hospital
Cambridge, Massachusetts, United States
Presenting Author(s)
Background: Congenital Anomalies of the Kidneys and Urinary Tract (CAKUT) are the leading cause of chronic kidney disease before 25 years of age. CAKUT has high morbidity, accounting for ~40% of childhood end-stage renal diseases. ENPP6 is a membrane-bound choline-specific glycerophospho-diesterase. Xenopus studies demonstrated impaired pronephros development upon Enpp6 knockdown. Pathogenic variants in the ENPP6 paralogue PIGN cause syndromic human CAKUT. Enpp6 KO mice excrete beta-glycerophosphocholine (beta-GPC) in their urine.
Objective: Identification of novel monogenic causes of CAKUT.
Design/Methods: We applied exome sequencing to our worldwide cohort of 2,423 individuals (probands, siblings, and parents) in 1,265 families with CAKUT. We analyzed existing Enpp6 X-ray crystal structure data and tested three ENPP6 variants identified in patients for enzymatic activity in vitro by establishing a fluorescent probe assay. Human patient urine was analyzed for choline metabolite, including beta-GPC using LC-MS.
Results: We identified a potentially deleterious homozygous missense variant (c.727A>G; p.M243V) in ENPP6 in a patient from a consanguineous family with posterior urethral valves and neurogenic bladder. Homozygous missense variants in ENPP6 are rare in gnomAD and a Saudi Arabian reference database. Residue p.M243 is part of a five amino acids-long motif (SDHGM) involved in coordinating two Zinc atoms that support substrate binding. The motif is evolutionarily conserved to S. cerevisiae and among the ENPP gene family (ENPP1–7) and the paralogue PIGN. We identified two additional families with homozygous ENPP6 variants and potential CAKUT phenotypes (c.1228G>A; p.V410M, and c.1088T>C; p.M363T). To quantify the enzymatic activity of identified mutants, we established a fluorescent probe assay showing that wildtype full-length human ENPP6 transiently overexpressed in HEK293 cells catalyzes the release of strongly fluorescent 2-Me-4OMe TG from a non- fluorescent synthetic probe (TG-mPC) consisting of the ENPP6 substrate phosphocholine linked to the fluorophore Tokyo green in a time and concentration-depended manner. Mutated ENPP6 p.M243V and p.M363T had reduced release of fluorescent 2-Me-4OMe TG compared to wildtype ENPP6. Next, we collected urine samples from the whole index family carrying the ENPP6 p.M243V allele and detected the phosphocholine metabolite beta-GPC via LC- MS exclusively in the urine of the affected homozygous boy. These findings are consistent with urine analysis on published Enpp6 knockout mice.
Conclusion(s): This study provides evidence that biallelic variants in ENPP6 can cause human CAKUT.
Training status certification_BCRP
PAS_2025_confirmation_training.pdf
Figure 2. hENPP6 fluorescent probe assay.
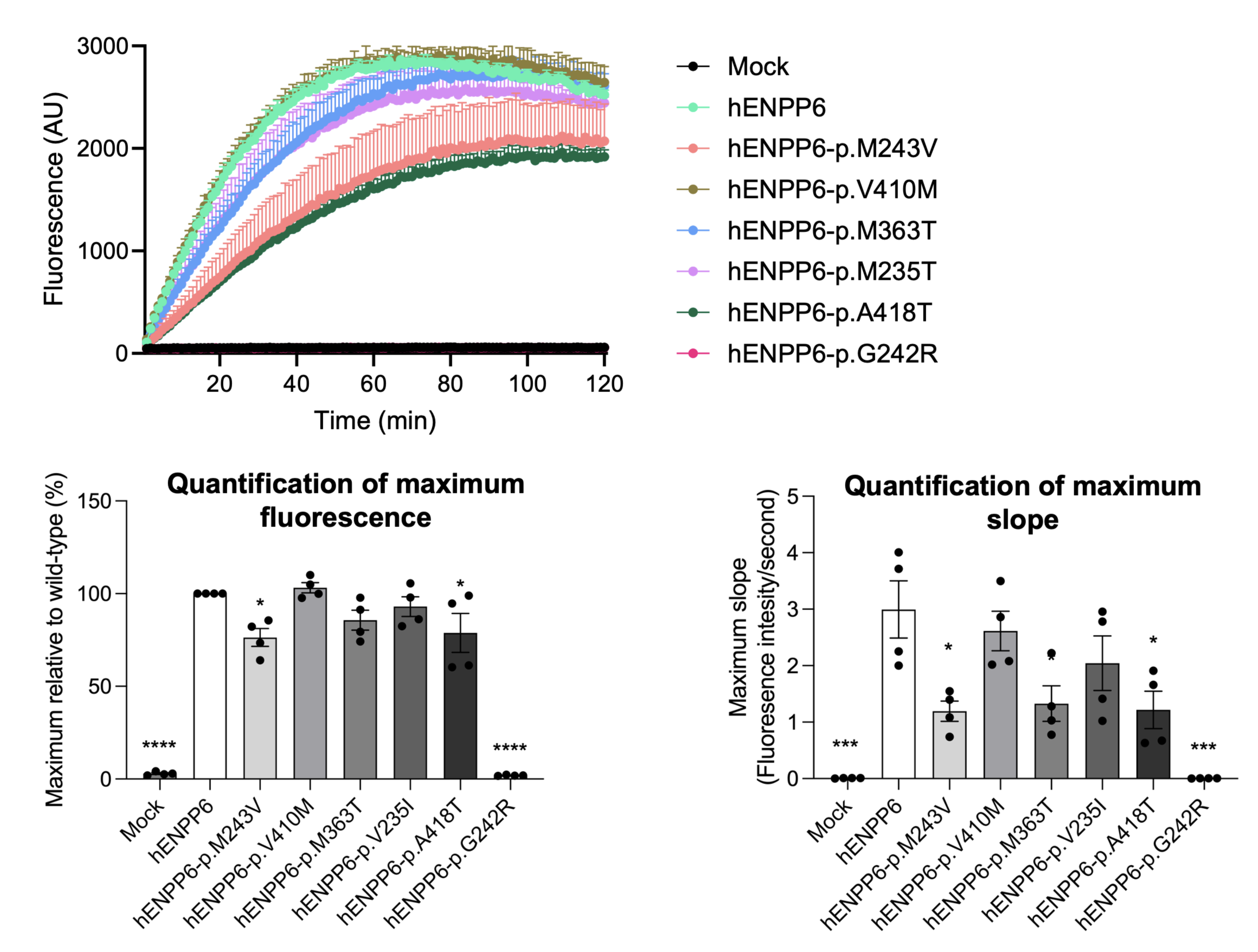 Graphs on the left depict the measured fluorescence over time after adding TG-mPC to HEK293 cells, overexpressing different human ENPP6 mutants. Bar graphs on the right show the statistical quantification of the maximum fluorescence and steepest rate of rise (One-way ANOVA) relative to the WT control.
Graphs on the left depict the measured fluorescence over time after adding TG-mPC to HEK293 cells, overexpressing different human ENPP6 mutants. Bar graphs on the right show the statistical quantification of the maximum fluorescence and steepest rate of rise (One-way ANOVA) relative to the WT control. Figure 4. LC-MS urine analysis of Index Family A for beta-GPC.
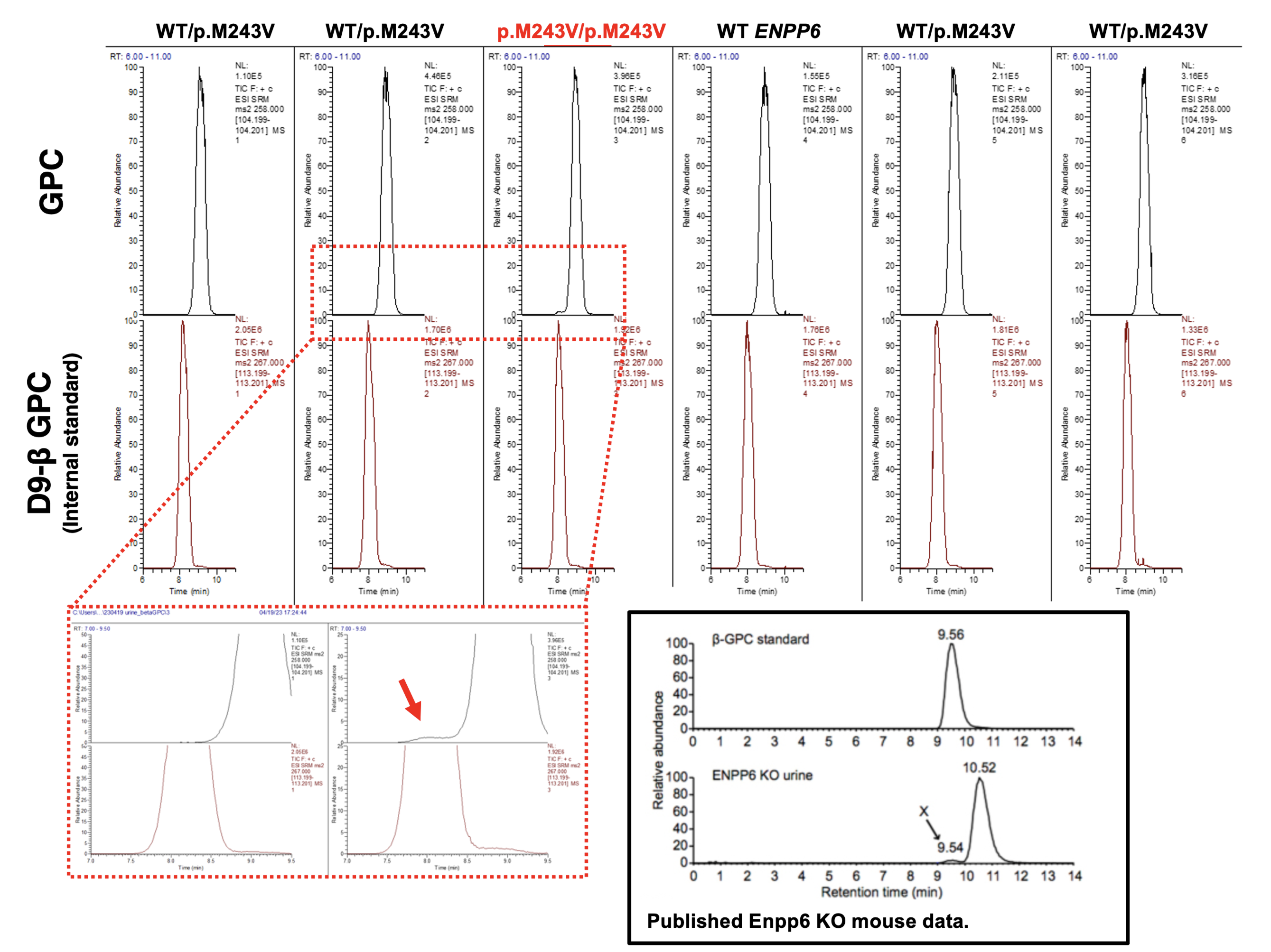 Black box on the lower right shows published data on the urinary excretion of beta-GPC found in Enpp6 KO mice (Morita et al., 2016).
Black box on the lower right shows published data on the urinary excretion of beta-GPC found in Enpp6 KO mice (Morita et al., 2016).
