Newborn Care 1
Session: Newborn Care 1
246 - Enhancing Early Detection of Biliary Atresia: A Quality Improvement Initiative at a Single Center
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 246.4758
Tebyan Rabbani, Lucile Packard Children's Hospital Stanford, Palo Alto, CA, United States; Janelle Aby, Stanford University School of Medicine, Palo Alto, CA, United States; Rachel Bensen, Stanford University School of Medicine, Palo Alto, CA, United States; Shannon Chan, Lucile Packard Children's Hospital Stanford, Palo Alto, CA, United States; Lauren Destino, Stanford University School of Medicine, Menlo Park, CA, United States; Adam Frymoyer, Stanford University School of Medicine, Palo Alto, CA, United States; Amrita Narang, Stanford University School of Medicine, Palo Alto, CA, United States

Tebyan Rabbani, DO (he/him/his)
Fellow
Lucile Packard Children's Hospital Stanford
Palo Alto, California, United States
Presenting Author(s)
Background: Biliary atresia (BA) is a severe pediatric liver disease characterized by obstruction of the extrahepatic biliary tree, affecting 1 in 8,000–18,000 births. BA is the leading cause of pediatric liver transplantation. Early identification and surgical intervention through the Kasai portoenterostomy (KPE) before 45 days of life (DOL) is associated with better outcomes. However, in the U.S. the average age at KPE is >60 DOL. Improved early detection protocols are needed.
Objective: To describe and evaluate the implementation of a 2-stage BA screening approach using serum direct bilirubin (DB) at a university-affiliated newborn nursery in California.
Design/Methods: Process mapping and current state analysis identified communication gaps and symptom misattribution contributing to delayed diagnosis. The 2-stage BA screening protocol was integrated into the nursery workflow, leveraging total serum bilirubin already collected at 24 hours of life. Infants with elevated DB levels in Stage 1 underwent repeat DB tests (Stage 2) with their primary care provider (PCP). Educational campaigns were conducted for nursery staff and PCPs, and a structured communication pathway was established for reporting positive screens. Regular stakeholder meetings facilitated continuous process refinement. A dedicated provider coordinated outpatient DB testing. We measured the percentage completing Stage 1, positives completing Stage 2 within 14-17 DOL, and the coordination effort.
Results: From April to October 2024, 2,513 infants born at ≥35 weeks gestation were admitted to the nursery. 2,502 (99.6%) completed Stage 1 screening, with 22 infants (0.87%) screening positive. Of those, 21 (95.4%) completed a repeat Stage 2 test, with no positive results. In Stage 2, 19 infants (86.4%) were screened within the target range of 14-17 DOL, with 3 infants experiencing delays in repeat testing due to order or transportation issues. During Stage 2, an average of 1.4 call attempts (SD1.1) were needed to reach parents, and 100% of PCPs were reached on the first call. Call duration averaged 7.9 minutes for parents and 2.3 minutes for PCPs. No infants had discharge delays due to screening.
Conclusion(s): High adherence to the 2-stage BA screening protocol was achieved through targeted education, structured communication pathways, process standardization, and stakeholder engagement. A dedicated provider facilitating follow-up was crucial to the protocol’s success, though the time requirements were minimal. Our systematic approach and implementation strategy offer a framework that can support adopting the 2-stage BA screening protocol in other centers.
Figure 1
.jpg) Workflow of biliary atresia screening using direct bilirubin
Workflow of biliary atresia screening using direct bilirubinFigure 2
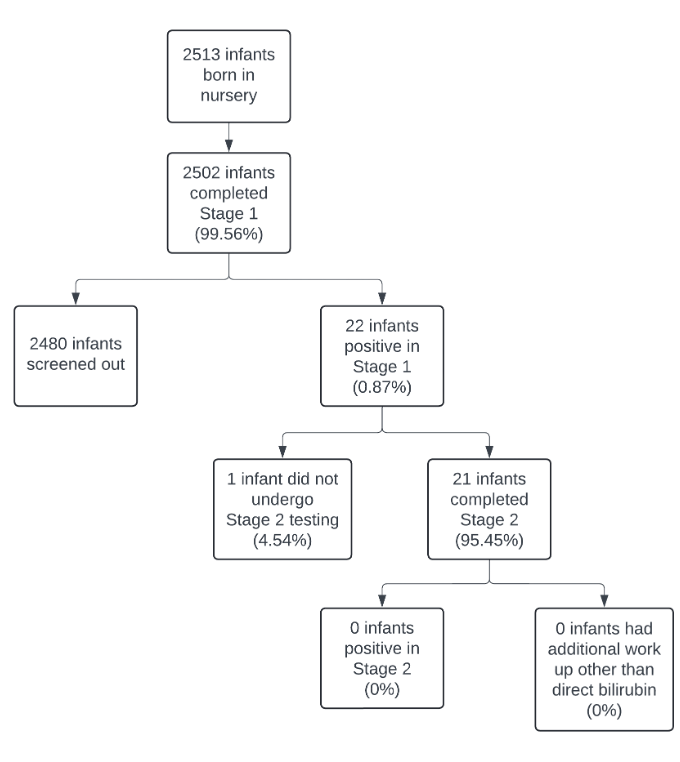 Flow chart depicting outcomes of biliary atresia screening
Flow chart depicting outcomes of biliary atresia screeningFigure 3
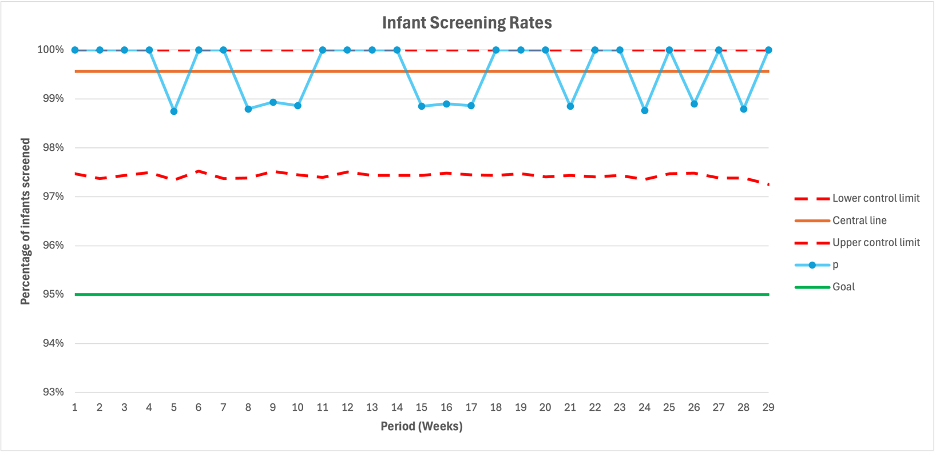 P chart depicting screening rates for biliary atresia
P chart depicting screening rates for biliary atresia

