Neonatal Neurology 4
Session: Neonatal Neurology 4
650 - Neonatal EEG-Based Machine Learning Models for Predicting Long-Term Antiseizure Medication Requirements and Epilepsy Diagnosis
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 650.5801
Lingkai Tang, Western University, London, ON, Canada; Kevin Kordbacheh, University of Ottawa Faculty of Medicine, Ottawa, ON, Canada; Emily Guarasci, London Health Sciences Centre, London, ON, Canada; Maryam Nabavi Nouri, Western University, London, ON, Canada; Emma G. Duerden, The University of Western Ontario - Schulich School of Medicine & Dentistry, London, ON, Canada
- LT
Lingkai Tang, ltang232@uwo.ca
Postdoctoral Associate
Western University
London, Ontario, Canada
Presenting Author(s)
Background: Newborns with neonatal encephalopathy are at high risk of having seizures after birth and can later develop epilepsy, which can have downstream effects on neurodevelopmental outcomes and quality of life. In clinical settings, prescribing antiseizure medication (ASM) upon discharge is common for newborns deemed at risk of ongoing seizures, although the decision-making process can be complex due to various influencing factors. Electroencephalography (EEG) is commonly used to monitor the newborn brain and can identify atypical abnormal brain activity that may precede or accompany seizures, allowing for timely intervention. In turn, early detection through EEG can be crucial for timely interventions. We propose that machine learning based predictive models, trained with early EEG data, can be a potential solution to the two issues.
Objective: Three predictive tasks were performed to examine whether features extracted from neonatal EEG, combined with machine learning methods, can predict the prescription of ASM on discharge and epilepsy at two later follow-ups, for patients having seizures right after birth.
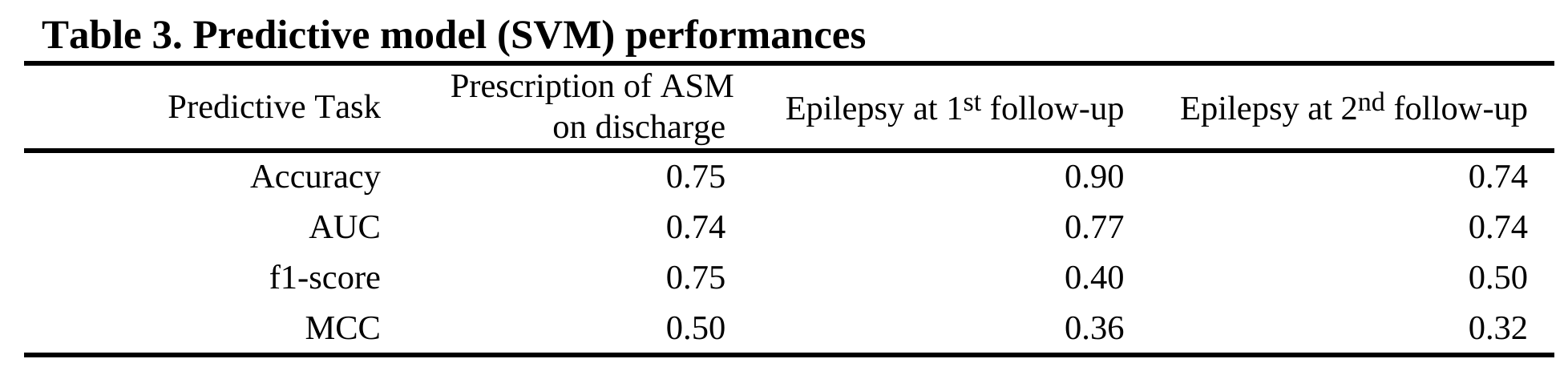
Design/Methods: In total, 78 newborns with hypoxic ischemic encephalopathy (HIE) participated. Due to missing data, not all participants completed all three predictive tasks (Table 1). EEG was acquired within days after birth with a sampling rate of 500 Hz for 20 minutes. For extracting features, a bilateral montage was applied with 8 channels, the recordings were filtered to 0.5 to 70 Hz, and a list of quantitative EEG features were extracted. Detailed montage and feature list can be found in Table 2. Support vector machine (SVM) models were trained with 5-fold validation to predict prescription of ASM on discharge and epilepsy at two follow-ups. Model performance was evaluated by accuracy, area under receiver operating characteristic curve (AUC), f1-score and Matthews correlation coefficient (MCC). The latter two metrics were better for unbalanced classes.
Results: The predictive model achieved above-chance performance for ASM prescription (Table 3). The f1-scores and MCCs for epilepsy prediction indicated the model can work with unbalanced classes. The results showed SVM models trained with the listed EEG features (Table 2) can perform the predictive tasks.
Conclusion(s): Our findings suggest that neonatal EEG data has potential to predict the prescription of ASM on discharge and later development of epilepsy. Future work includes acquiring more samples, feature engineering for better performance, and importance ranking of EEG features for clearer clinical interpretation and decision making.
Table 1. Participant demographics.
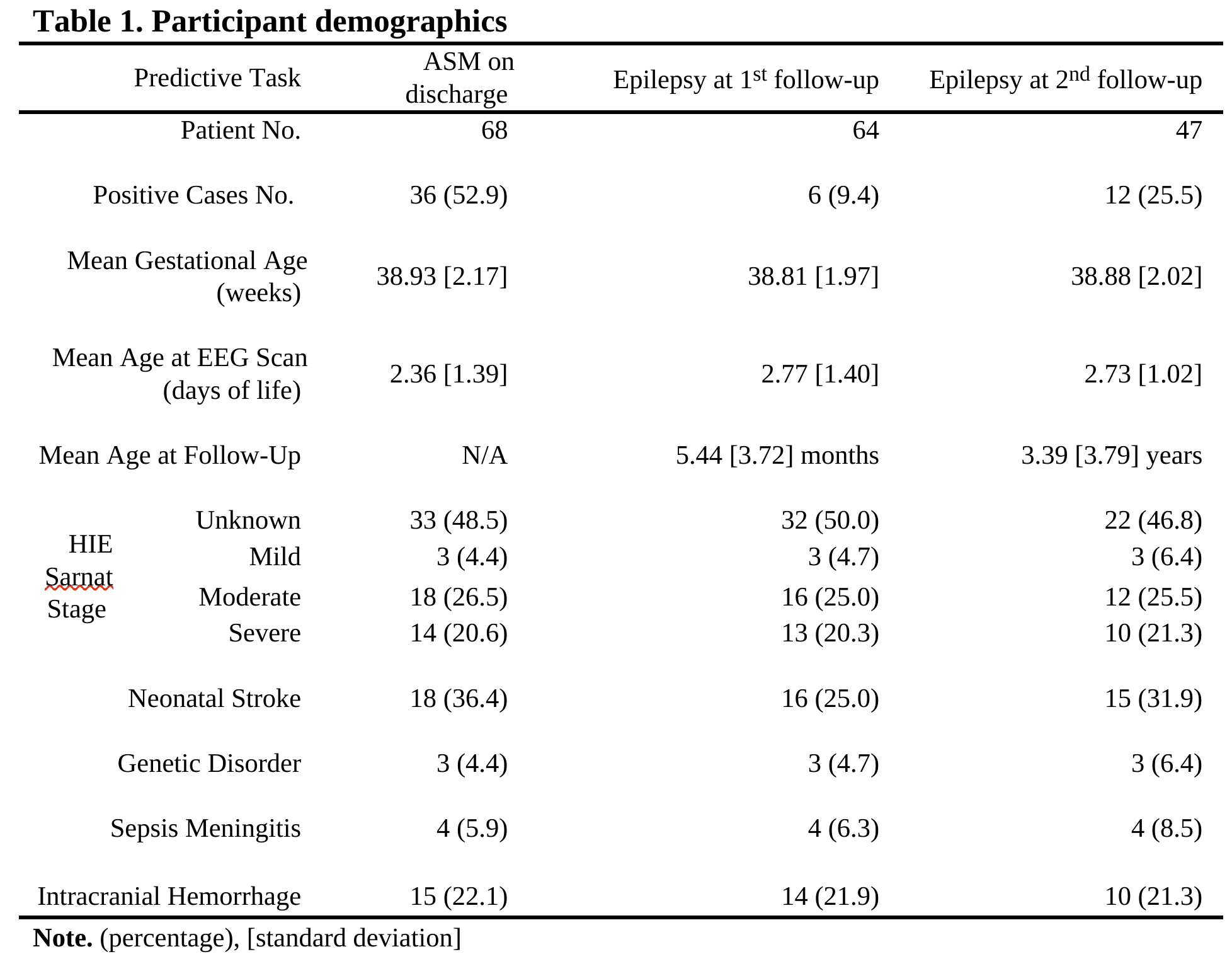
Table 2.
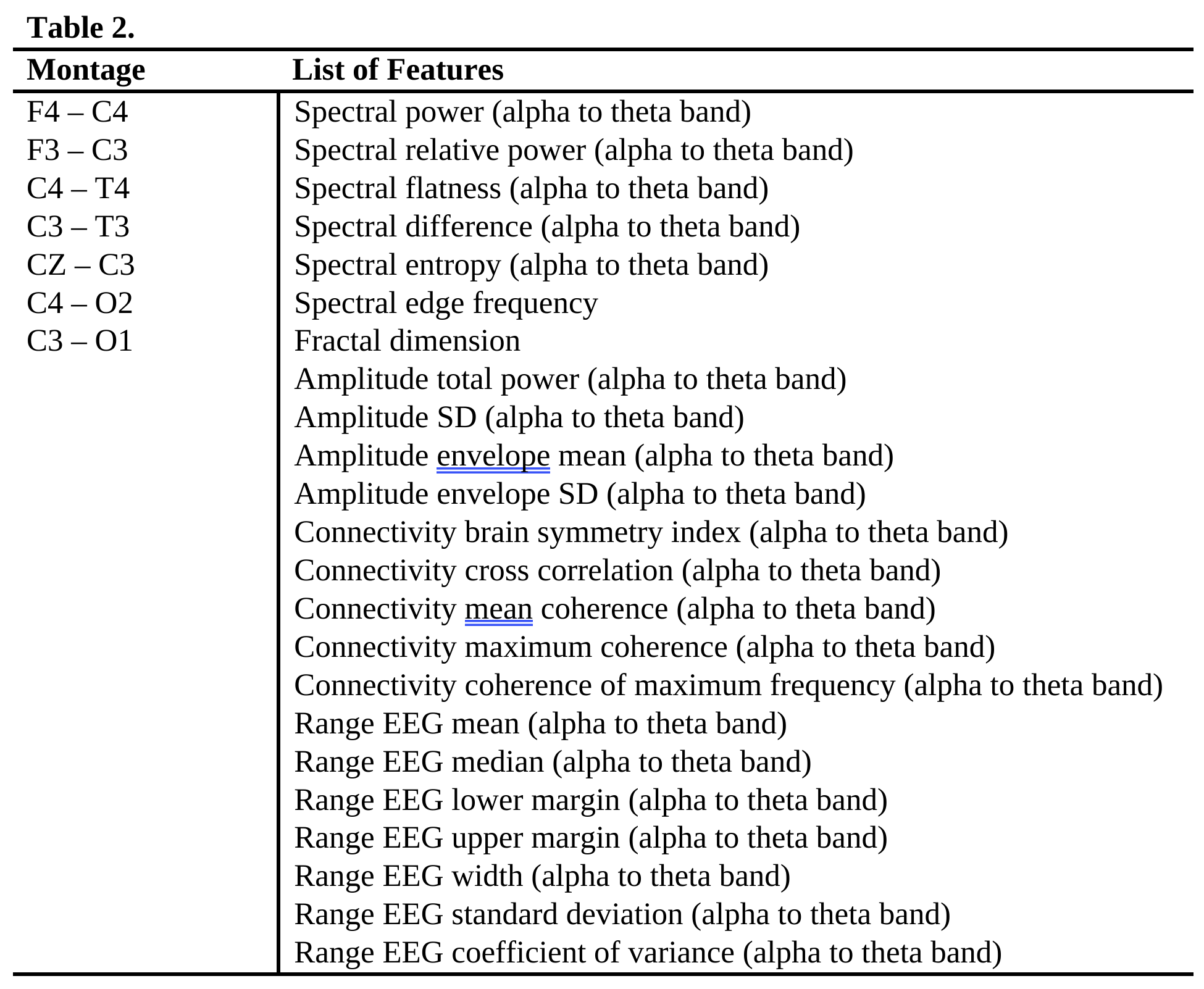
Table 3. Predictive model (SVM) performances