Nephrology 2
Session: Nephrology 2
020 - Methods of a Pilot Clinical Trial: Transcutaneous Vagus Nerve Stimulation for the Treatment of Childhood Nephrotic Syndrome
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 20.5949
Christine Sethna, Cohen Childrens Medical Center, Northwell, New Hyde Park, NY, United States; Kristal Wong, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Westbury, NY, United States; Meghana Karan, Cohen Children's Medical Center, New York, NY, United States; Matthew Schuchman, Cohen Children's Medical Center, New Hyde Park, NY, United States; Mahie M. Abdullah, Cohen Children's Medical Center, Bronx, NY, United States; Suzanne Vento, Cohen Children's Medical Center, New Hyde park, NY, United States; Arielle Swenson. Nickel, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Kevin E. Meyers, Childrens Hospital of Philadelphia, Philadelphia, PA, United States
- CS
Christine Sethna, MD EdM
Division Chief, Pediatric Nephrology
Cohen Children's Medical Center
New Hyde Park, New York, United States
Presenting Author(s)
Background: Children with nephrotic syndrome, especially those that have frequent relapses (FRNS) or are resistant to steroid therapy (SRNS), are exposed to prolonged courses of steroids and other immunosuppressant medications. Given the adverse side effect profiles and variable efficacy of these medications, there is an urgent need to identify novel and safe therapies to treat nephrotic syndrome in children. Stimulation of the vagus nerve, which can be activated non-invasively by transcutaneous auricular vagus nerve stimulation (taVNS), modulates the immune system by the inflammatory reflex and spleen. taVNS has become a therapy of interest for treating chronic immune-mediated illnesses. The central hypothesis is that activation of the inflammatory reflex via taVNS modulates the immune response, attenuates relapses and proteinuria, and reduces reliance on immunosuppressant medications in children with nephrotic syndrome.
Objective: To conduct pilot clinical trials to evaluate the feasibility and tolerability of taVNS for the treatment of nephrotic syndrome in children and generate preliminary data to guide power calculations for a future larger study.
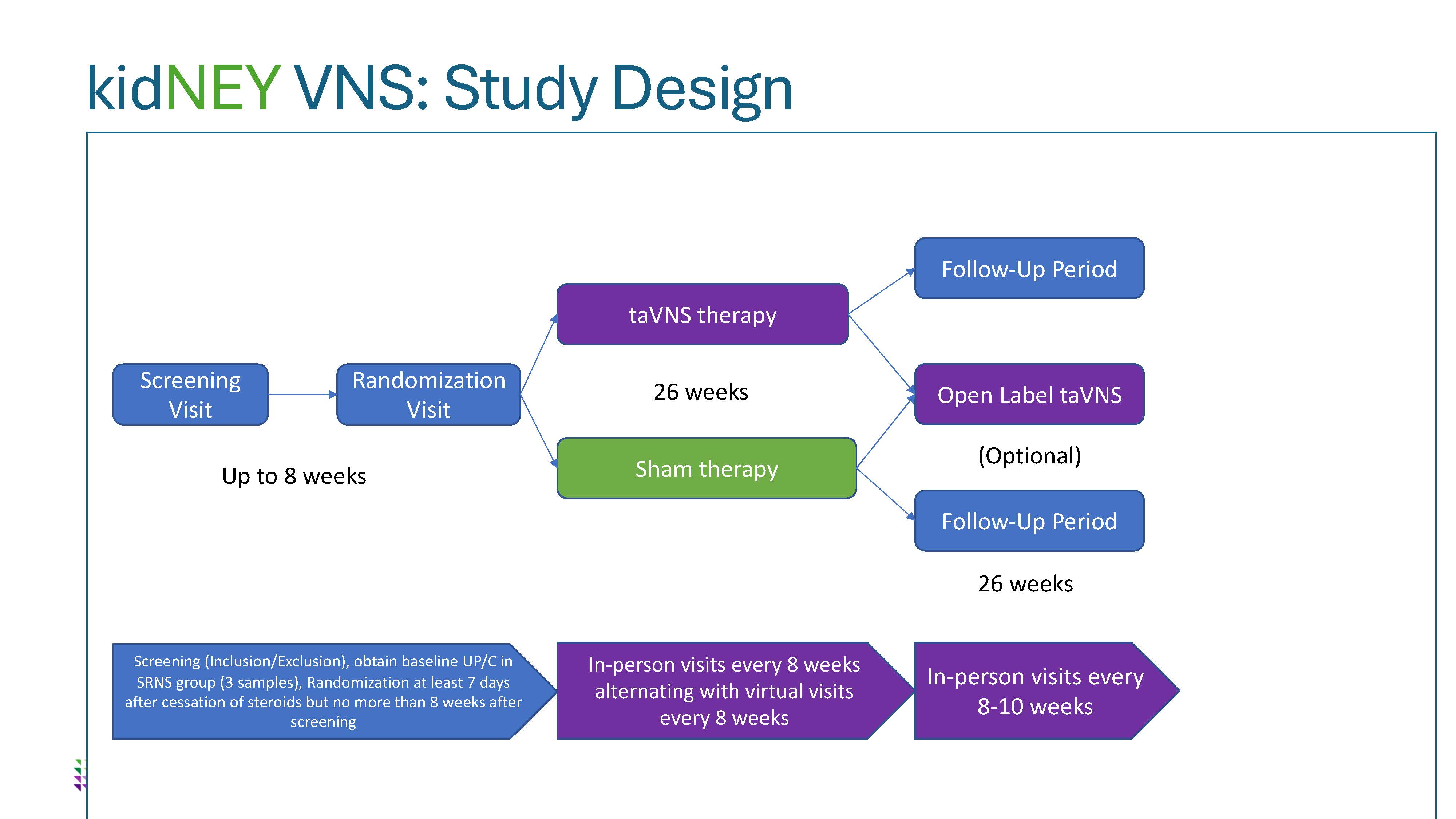
Design/Methods: This NIH-funded pilot study will enroll 30 children with FRNS and 10 children with SRNS age 3-17 years from two sites in two parallel, double-blinded, randomized placebo-controlled trials comparing five minutes of daily taVNS use with sham therapy for 26 weeks. To test for feasibility and tolerability of the clinical trial, the process of recruitment, adherence, device functionality, time and resource utilization, and safety monitoring will be examined. Using pre-specified proof-of-concept criteria, preliminary data gathered from this pilot study will then be used to determine whether a subsequent larger main trial should move forward. Further, immune markers including serum and monocyte-stimulated cytokines and anti-nephrin antibodies will be measured in order to characterize the immunomodulatory effect of taVNS therapy.
Results: The study is currently enrolling participants.
Conclusion(s): Accomplishing the goals of this pilot study will provide preliminary data that will inform the design of and strengthen the implementation of a planned larger clinical trial. Providing these preliminary data that are essential to conduct a large-scale trial will address a significant knowledge gap and will pave the way for evaluation of an innovative, non-pharmacologic, non-invasive, steroid-sparing approach to the treatment of idiopathic nephrotic syndrome in children.
taVNS Pilot Trial Study Design
.jpg)
taVNS Pilot Trial Study Schedule