Back
Background: Opioid replacement therapy is indicated if signs of neonatal opioid withdrawal syndrome (NOWS) persist after non-pharmacologic therapy. Opioid weaning is the longest phase of this therapy and lacks an evidence-based approach.
Objective: Examine whether a rapid wean protocol (15% reductions) is tolerated and reduces days of opioid treatment compared to a slow wean protocol (10% reductions) among newborns receiving morphine or methadone as primary pharmacological treatment for NOWS.
Design/Methods: A pragmatic, blinded, randomized multicenter trial was conducted among newborns treated with opioid for NOWS (9/20-12/23) in 21 US hospitals of the Advancing Clinical Trials in Neonatal Opioid Withdrawal Consortium. Infants were ≥ 36 weeks gestation and receiving morphine or methadone as primary treatment for NOWS. After stabilization of NOWS signs, newborns were individually randomized to protocol weaning of 8 decreasing opioid dose levels with reductions of 15% or 10% (rapid or slow wean respectively). Weaning was encouraged every 24h and if not tolerated, the preceding dose was resumed. To maintain blinding, the last 3 dose levels for rapid wean were placebo. Hospitals continued all other usual practices for NOWS care. The primary outcome was days of opioid treatment from first weaning dose to cessation of opioids. Intent to treat analysis used linear mixed models reporting results as adjusted means and 95% confidence intervals (CI).
Results: 189 newborns were randomized, 98 (52%) to rapid wean (mean±sd, 38.8±1.2 weeks gestation, 59.8% male) and 91 (48%) to slow wean (38.8±1.3 weeks gestation, 61.5% male). Morphine was used most commonly in the rapid and slow wean groups (80.6 and 72.5%, respectively). Weaning started at 5.8±2.5 and 6.3±3.0 days for the rapid and slow wean groups, respectively (Table 1). Intent to treat analysis included all but 4 withdrawn newborns in the rapid wean group. Adjusted mean days (95% CI) of opioid treatment during weaning were reduced for rapid vs slow wean groups: 8.2 (7.2, 9.5) vs 11.2 (9.6, 12.9) respectively, p< 0.001) with a mean difference of 3.0 days (1.7, 4.3) (Table 2). Adverse events were rare in both groups. Neonatal neurobehavioral assessments (NNNs-II) prior to discharge were similar between groups. Results for secondary outcomes were consistent (Table 3).
Conclusion(s): Pharmacologic treatment of NOWS using weaning decrements of 15% were well tolerated and resulted in fewer days of opioid treatment compared to 10% decrements. This strategy can reduce days of opioid treatment for pharmacologic therapy of NOWS.
Table 1: Newborn Characteristics
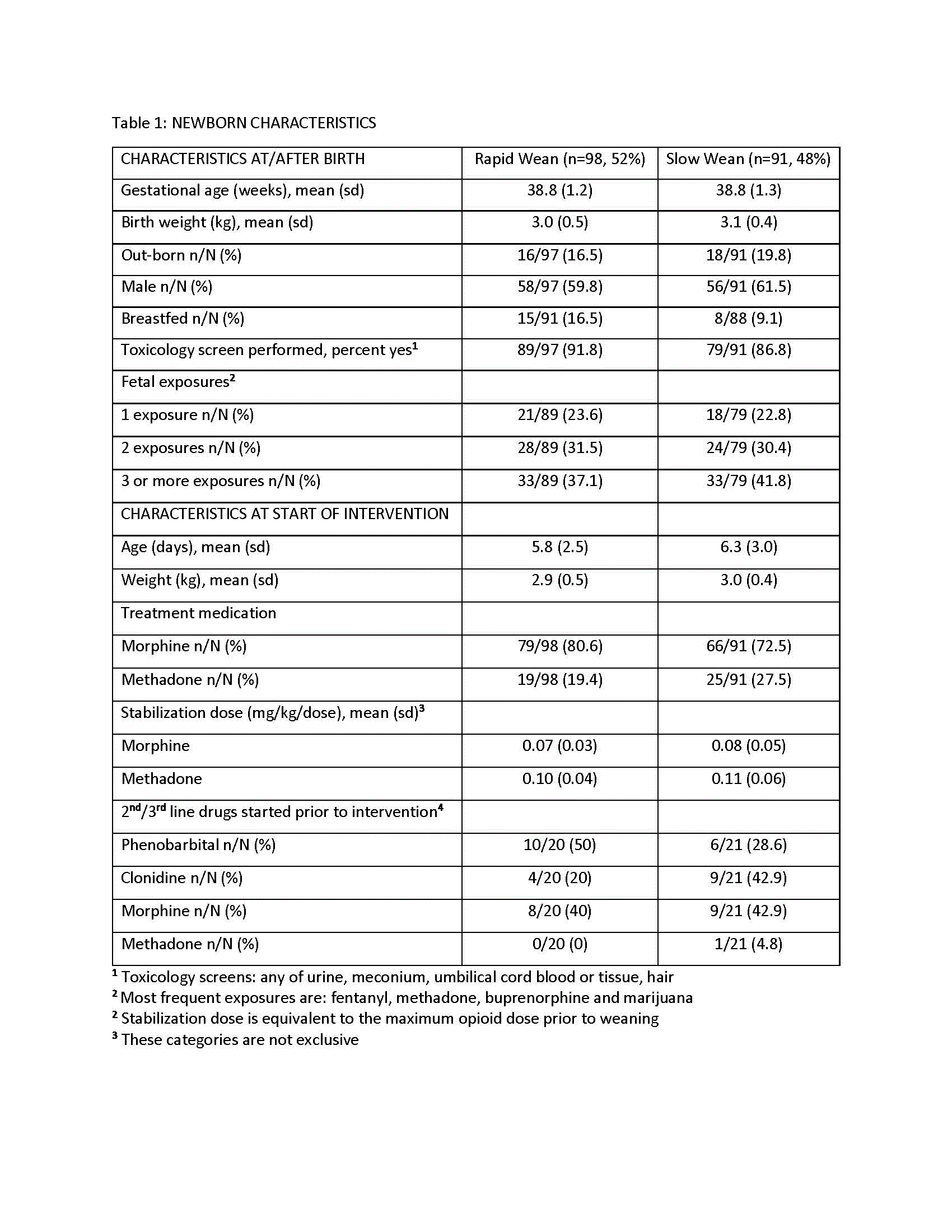
Table 2: Primary Outcome
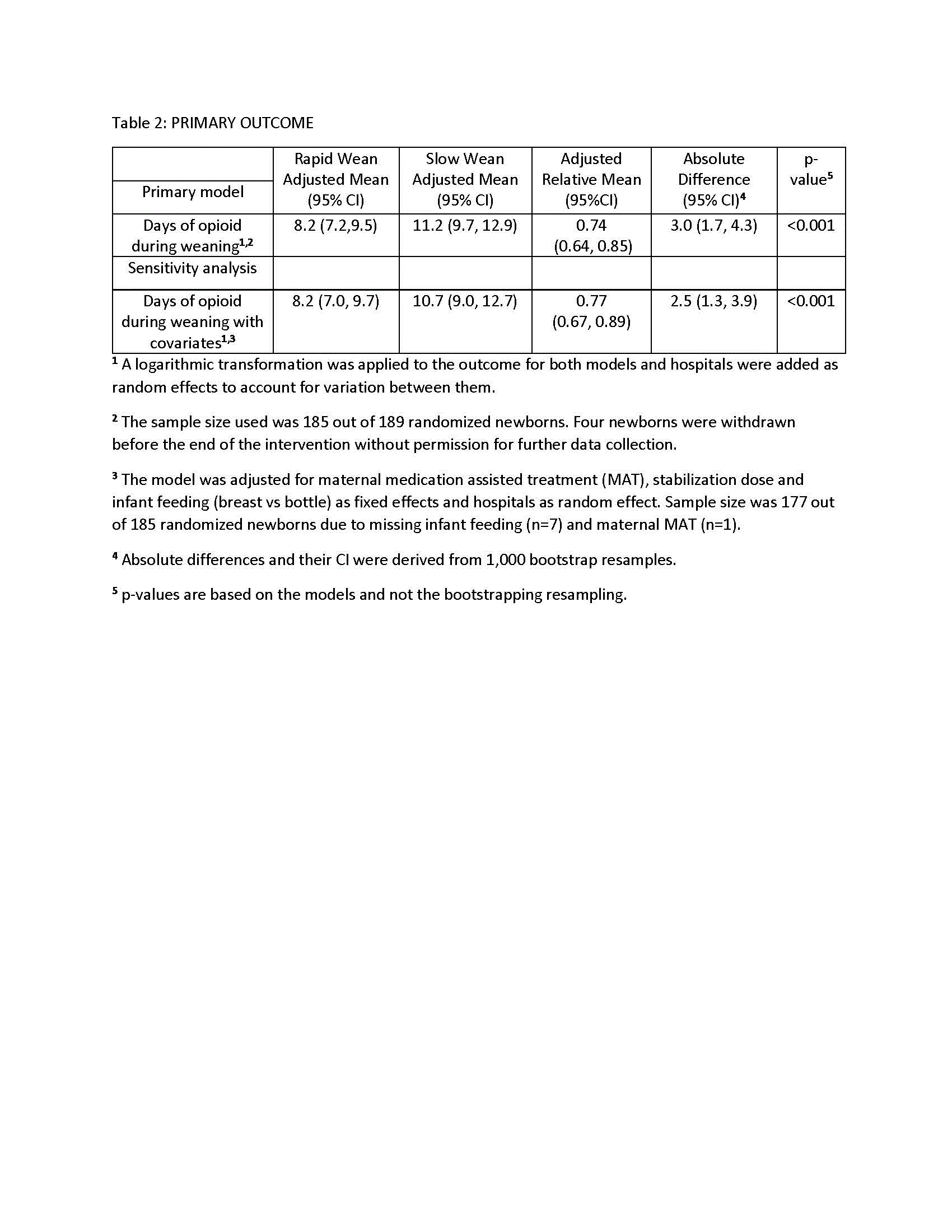
Table 3: Secondary Outcomes
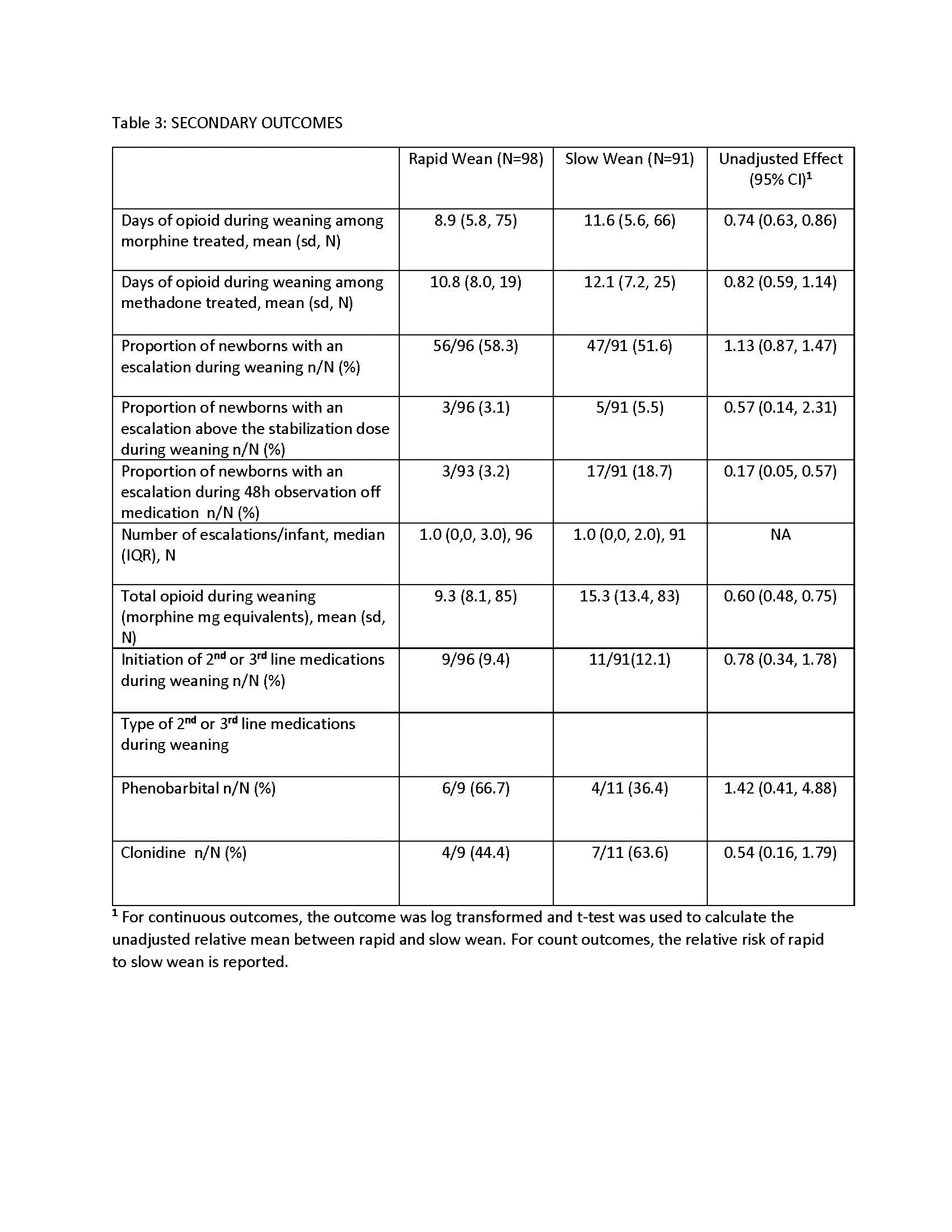
Table 1: Newborn Characteristics

Table 2: Primary Outcome

Table 3: Secondary Outcomes

Neonatal Clinical Trials 1
Session: Neonatal Clinical Trials 1
556 - A Multicenter Randomized, Blinded Trial of Weaning Opioids for Neonatal Opioid Withdrawal Syndrome
Sunday, April 27, 2025
8:30am – 10:45am HST
Abbot Laptook, Women & Infants Hospital of Rhode Island, Providence, RI, United States; Adam Czynski, Connecticut Children's Medical Center, East Greenwich, RI, United States; Rouba A. Chahine, RTI International, Vestavia, AL, United States; Rachel G. Greenberg, Duke Clinical Research Institute, Durham, NC, United States; Brian Smith, Duke University, Durham, NC, United States; Erica L. Oliveira, WIHRI, Providence, RI, United States; Jenna Gabrio, RTI, Livermore, CA, United States; Barry S. Eggleston, RTI International, Mebane, NC, United States; Abhik Das, RTI International, North Potomac, MD, United States; Jeannette Lee, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Barry Lester, The Warren Alpert Medical School of Brown University, Providence, RI, United States; Dave Clark, NIH/NICHD, Bethesda, MD, United States; Jessica Snowden, Univ of Tennessee Health Science Center, Memphis, TN, United States; NICHD Neonatal Research Network, National Institute of Child Health and Human Development, Bethesda, MD, United States; ECHO IDeA States Pediatric Clinical Trials Network, n/a, North Bethesda, MD, United States

Abbot Laptook, MD
Emeritus Professor of Pediatrics
Women & Infants Hospital of Rhode Island
Providence, Rhode Island, United States
Presenting Author(s)
Background: Opioid replacement therapy is indicated if signs of neonatal opioid withdrawal syndrome (NOWS) persist after non-pharmacologic therapy. Opioid weaning is the longest phase of this therapy and lacks an evidence-based approach.
Objective: Examine whether a rapid wean protocol (15% reductions) is tolerated and reduces days of opioid treatment compared to a slow wean protocol (10% reductions) among newborns receiving morphine or methadone as primary pharmacological treatment for NOWS.
Design/Methods: A pragmatic, blinded, randomized multicenter trial was conducted among newborns treated with opioid for NOWS (9/20-12/23) in 21 US hospitals of the Advancing Clinical Trials in Neonatal Opioid Withdrawal Consortium. Infants were ≥ 36 weeks gestation and receiving morphine or methadone as primary treatment for NOWS. After stabilization of NOWS signs, newborns were individually randomized to protocol weaning of 8 decreasing opioid dose levels with reductions of 15% or 10% (rapid or slow wean respectively). Weaning was encouraged every 24h and if not tolerated, the preceding dose was resumed. To maintain blinding, the last 3 dose levels for rapid wean were placebo. Hospitals continued all other usual practices for NOWS care. The primary outcome was days of opioid treatment from first weaning dose to cessation of opioids. Intent to treat analysis used linear mixed models reporting results as adjusted means and 95% confidence intervals (CI).
Results: 189 newborns were randomized, 98 (52%) to rapid wean (mean±sd, 38.8±1.2 weeks gestation, 59.8% male) and 91 (48%) to slow wean (38.8±1.3 weeks gestation, 61.5% male). Morphine was used most commonly in the rapid and slow wean groups (80.6 and 72.5%, respectively). Weaning started at 5.8±2.5 and 6.3±3.0 days for the rapid and slow wean groups, respectively (Table 1). Intent to treat analysis included all but 4 withdrawn newborns in the rapid wean group. Adjusted mean days (95% CI) of opioid treatment during weaning were reduced for rapid vs slow wean groups: 8.2 (7.2, 9.5) vs 11.2 (9.6, 12.9) respectively, p< 0.001) with a mean difference of 3.0 days (1.7, 4.3) (Table 2). Adverse events were rare in both groups. Neonatal neurobehavioral assessments (NNNs-II) prior to discharge were similar between groups. Results for secondary outcomes were consistent (Table 3).
Conclusion(s): Pharmacologic treatment of NOWS using weaning decrements of 15% were well tolerated and resulted in fewer days of opioid treatment compared to 10% decrements. This strategy can reduce days of opioid treatment for pharmacologic therapy of NOWS.
Table 1: Newborn Characteristics

Table 2: Primary Outcome

Table 3: Secondary Outcomes

Table 1: Newborn Characteristics

Table 2: Primary Outcome

Table 3: Secondary Outcomes


