Infectious Diseases 5: In utero exposures and infections
Session: Infectious Diseases 5: In utero exposures and infections
037 - Increasing Testing for Congenital Cytomegalovirus Infection in Newborns with Sensorineural Hearing Loss: A Quality Improvement Project.
Sunday, April 27, 2025
8:30am - 10:45am HST
Publication Number: 37.5377
Samantha Lonergan, Madigan Army Medical Center, Tacoma, WA, United States; Rebecca Sainato, Madigan Army Medical Center, Tacoma, WA, United States; Blake Cirks, Madigan Army Medical Center, Joint Base Lewis McChord, WA, United States; Brendan Carty, Unknown, Philadelphia, PA, United States; Jacob Hogue, Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine, Tacoma, WA, United States; Emily Parsons, Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine, Bethesda, MD, United States; Joshua DeJong, Madigan Army Medical Center, Tacoma, WA, United States; Katie M. Teague, Madigan Army Medical Center, DHA, DoD, Tacoma, WA, United States
- SL
Samantha Lonergan, MD (she/her/hers)
Resident
Madigan Army Medical Center
Tacoma, Washington, United States
Presenting Author(s)
Background: Congenital cytomegalovirus infection (cCMV) is the leading non-genetic cause of sensorineural hearing loss (SNHL). Early antiviral treatment has been shown to improve audiologic outcomes in those with isolated SNHL, but testing to confirm cCMV must be completed ≤21 days of birth. Many states now require CMV testing for neonates with SNHL, but there are high rates of method variability and success in achieving this metric.
Objective: This Quality Improvement (QI) project goal is to test all neonates with SNHL for CMV by 21 days.
Design/Methods: This multidisciplinary project is ongoing since January 1, 2019 at a single tertiary care center that delivers >1000 newborns annually and provides specialty care, including audiology visits, for more than 25,000 pediatric patients. It uses the Model for Improvement framework with Plan-Do-Study-Act cycles. Interim causal analysis identified delayed evaluations for SNHL by audiology and coordination of CMV sample collection in those with SNHL as key drivers. The most recent cycle was initiated when saliva CMV polymerase chain reaction (PCR) testing became available at this center and replaced urine as the preferred sample. Simultaneously, this center also began testing all newborns who referred on nursery hearing screens for CMV.
Results: Table 1 shows the collected results since initiating this project and through September 30, 2024. During the latest cycle, the % neonates with failed SNHL, who are being screened for cCMV, increased from 43% to 63% (P= 0.298); all those who were identified with SNHL ≤21 days were screened for CMV. The change in key driver performance, as defined in methods, are displayed in a Run Chart (Figure 2). Missed opportunities are now primarily attributed to delays in obtaining Audiology exams, particularly for those delivered at outside hospitals. So far, this project has identified three infants with cCMV, including two in the most recent cycle. 2/3 were found to have symptomatic cCMV and were treated with antiviral therapy; one was asymptomatic without SNHL. After implementing salivary CMV testing for referred nursery hearing screens, the volume of CMV testing being performed increased 10-fold.
Conclusion(s): This project models some of the challenges and potential improvements to identify cCMV as a cause for SNHL, and can be useful in identification of those with symptomatic cCMV. Although more tests are being performed, only one infant with asymptomatic CMV was identified. More cycles are needed to determine the costs and benefits of screening more neonates, including those who are ultimately asymptomatic, for cCMV.
Table 1. Audiology and CMV Screening Results by Year
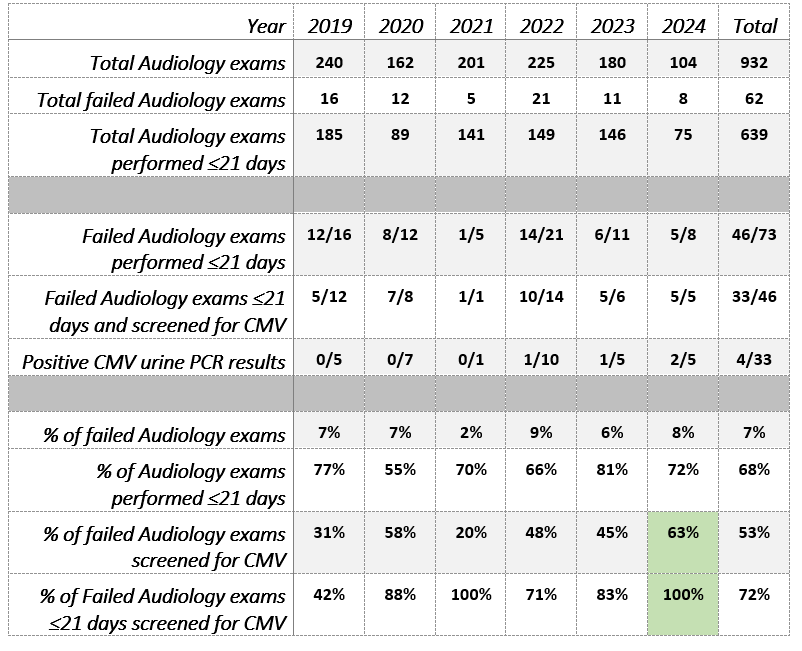
Figure 1. Timeline of Interventions with ach Plan-Do-Study-Act Cycle
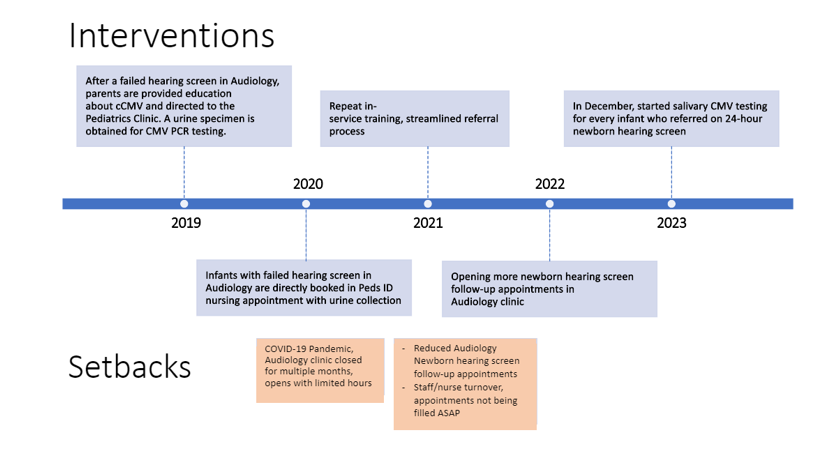
Figure 2. Run Chart of Missed Opportunities and CMV Candidates Successfully Tested
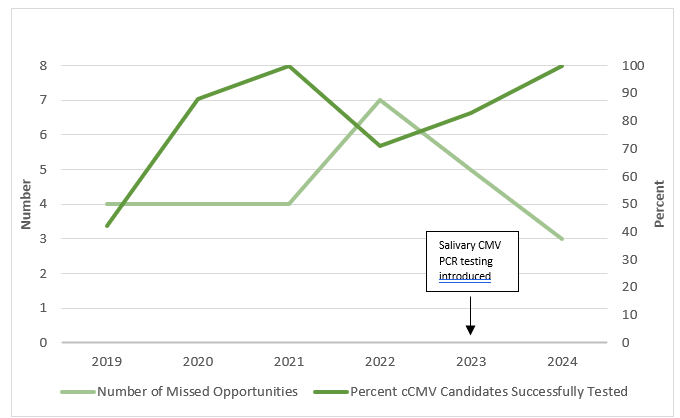 Missed opportunities are defined as neonates who failed audiology testing after 21 days and were not screened for CMV. CMV candidates included those who received audiology testing within 21 days.
Missed opportunities are defined as neonates who failed audiology testing after 21 days and were not screened for CMV. CMV candidates included those who received audiology testing within 21 days. 
